Olanzapine orally disintegrating tablet and preparation method thereof
A technology of orally disintegrating tablets and olanzapine, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of inconvenience for patients to take, unfavorable clinical medication, stability, etc. Poor quality and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
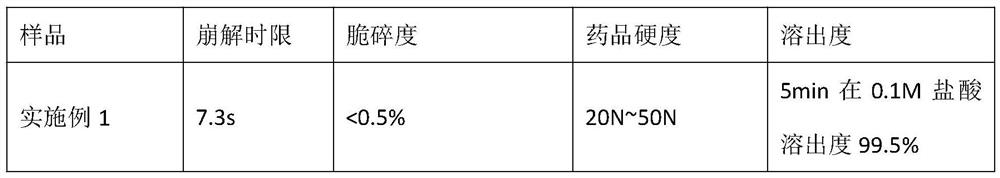
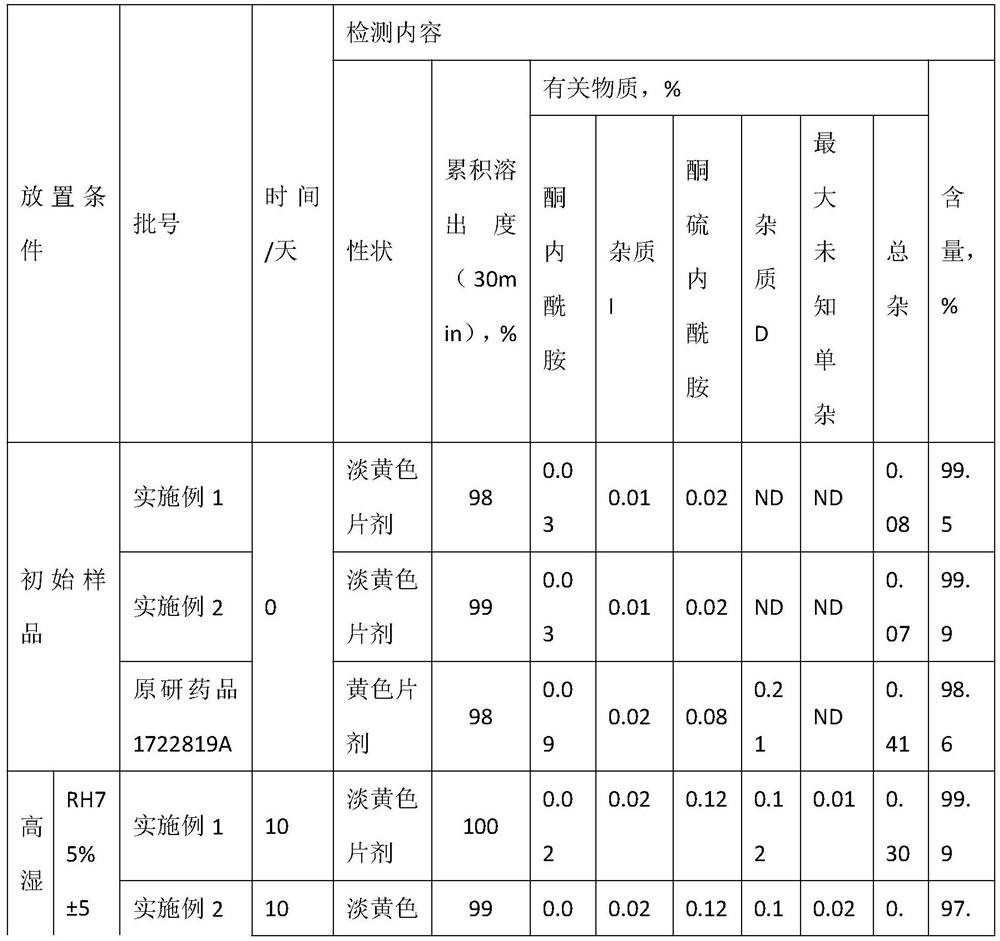
Embodiment 1
[0039] A method for preparing olanzapine composition, production of 10,000 sheet, comprising the steps of:
[0040] (1) Weigh: a weight ratio of each raw material was weighed and accessories, olanzapine 50g. Microcrystalline cellulose 250g, mannitol 90g, fructose 75g, colloidal silicon dioxide 10g, cross-linked polyvinyl pyrrolidone 20g, 5g magnesium stearate; wherein olanzapine particle size in D 90 Is 4μm-100μm; particle size of microcrystalline cellulose in D 90 Less than 50μm.
[0041] (2) sieving: the formulation amounts of fructose, colloidal silica, mannitol, crospovidone, microcrystalline cellulose through a 60 mesh sieve has, mixer set at 15 rpm / min, and mixed for 30 minutes, unloading.
[0042] Upon the addition of 200g of (2) was mixed together; (3) The formulation amounts of olanzapine (2) mixing calm 50g, the 80 mesh sieve; upon the addition of (2) was mixed together 100g of 80-mesh sieve , over 80 mesh sieve.
[0043] (4) mixing: the step (3) the resulting material...
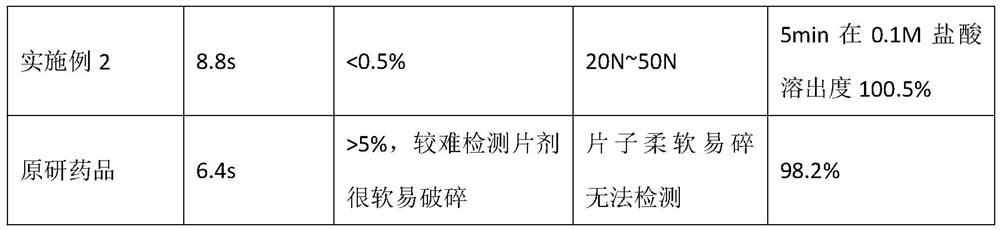
Embodiment 2
[0049] A method for preparing olanzapine composition, production of 10,000 sheet, comprising the steps of:
[0050] (1) Weigh: a weight ratio of each raw material was weighed and accessories, olanzapine 100g. Microcrystalline cellulose 200g, mannitol 90g, fructose 75g, colloidal silicon dioxide 10g, cross-linked polyvinyl pyrrolidone 20g, 5g magnesium stearate; wherein olanzapine particle size in D 90 Is 4μm-100μm; particle size of microcrystalline cellulose in D 90 Less than 50μm.
[0051] (2) sieving: the formulation amounts of fructose, colloidal silica, mannitol, crospovidone, microcrystalline cellulose through a 60 mesh sieve has, blender set at 20 rev / min, and mixed for 20 minutes, unloading.
[0052] (2) mixing (3) The formulation of olanzapine amount of 100g, 80 mesh sieve; continue after (2) the mixing 200g mixed together was added, over 80 mesh sieve.
[0053] (4) mixing: the step (3) the resulting material added to the mixer, the remaining mixture of step (2) the resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com