Application of attenuated yellow disease virus in oncolysis
A yellow fever virus and virus technology, which is applied in the field of biomedicine and achieves the effect of wide application prospect and small toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Embodiment 1: Construction and virus rescue of flavivirus attenuated strain clone
[0112] 1. Cloning construction of flavivirus attenuated strain
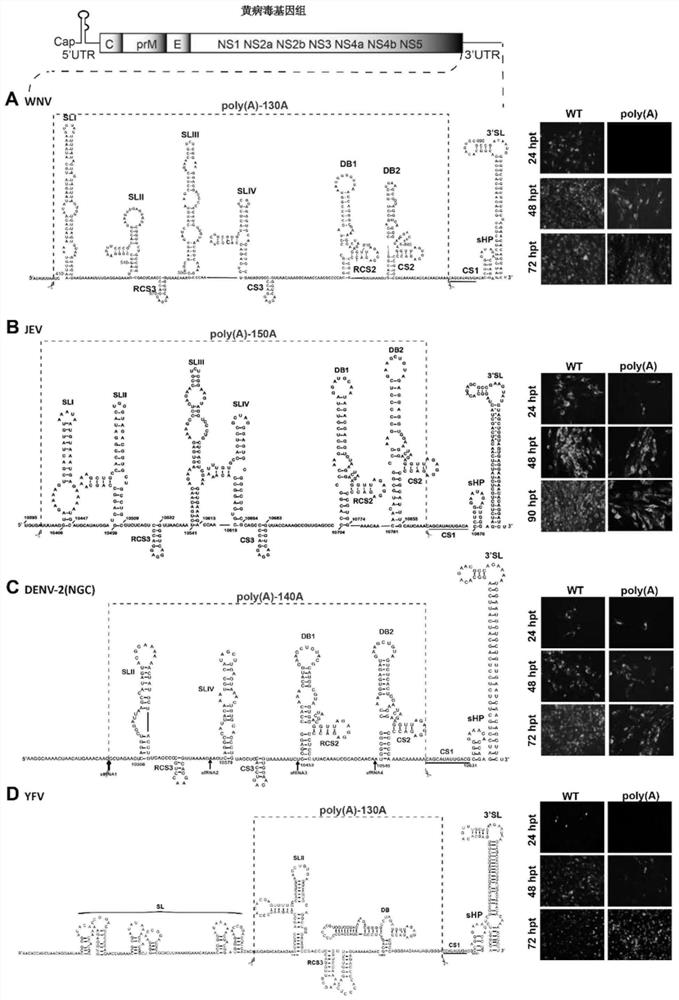
[0113] Schematic diagram of the cloning construction of attenuated flavivirus strains figure 1 shown.
[0114] Using the plasmid DNA of the infectious clone of the wild-type (WT) West Nile virus (WNV) strain as the backbone, the sequence from SLI to CS2 (including the 5' Terminal stem-loop region I (SLI), stem-loop region II (SLII), repeat circularization sequence region 3 (RCS3), stem-loop region III (SLIII), stem-loop region IV (SLIV), circularization sequence region 3 (CS3 ), dumbbell region 1 (DB1), repeat circularizing sequence region 2 (RCS2), dumbbell region (DB2), circularizing sequence region 2 (CS2)) deletion, retaining circularizing sequence region 1 (CS1) in the 3' untranslated region ) and the short hairpin-3' stem-loop region (sHP-3'SL) after circularizing sequence region 1, and inserting a poly(A) sequence...
Embodiment 2
[0131] Example 2: Oncolytic effect of WNV-poly(A) on tumor cells in vitro
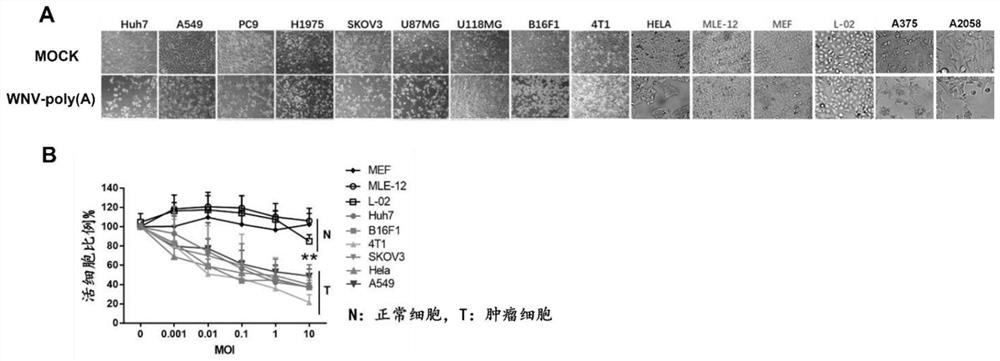
[0132] In this embodiment, after infecting tumor cells with the WNV-poly(A) virus attenuated virus (WNV-poly(A)) prepared in Example 1 with a fixed MOI dose, observe its killing effect on tumor cells in bright field ( figure 2 A), and CCK8 was used to detect the killing of tumor cells by different doses of WNV-poly(A) to determine the oncolytic effect of the virus in vitro ( figure 2 B). in:
[0133] Control group (MOCK): tumor cell group cultured in medium without virus;
[0134] Experimental group: the tumor cell group cultured in the medium added with WNV-poly(A).
[0135] 1. Bright field observation of the oncolytic effect of WNV-poly(A) on tumor cells and normal cells
[0136] 12 kinds of tumor cells Huh7, A549, PC9, H1975, SKOV3, U87MG, U118MG, B16F1, 4T1, HELA, A375 and A2058 and 3 kinds of normal cells MLE-12, MEF and L-02 were divided into 8×10 4 Cells / well were seeded in 12-well cell c...
Embodiment 3
[0142] Example 3: The killing difference of WNV-wT and WNV-poly(A) on normal cells and tumor cells in vitro
[0143] This embodiment is obtained by transfecting the wild-type West Nile virus (WNV-WT by WNV infectious clone 3356 strain) and the WNV-poly (A) virus (WNV-poly) virus prepared in Example 1 with a fixed MOI dose. (A) After infecting tumor cells, its killing effect on normal cells and tumor cells was observed in bright field ( image 3 A), and use CCK8 to detect the killing of different doses of WNV-WT and WNV-poly(A) to normal cells and tumor cells to determine the difference in killing of WNV-WT and WNV-poly(A) to normal cells and tumor cells, further The killing specificity of WNV-poly(A) to tumor cells was determined by comparing the growth curves of WNV-WT and WNV-poly(A) on normal cells and tumor cells ( image 3 B). in:
[0144] Control group (MOCK): tumor cell group cultured in medium without virus;
[0145] Experimental group: the tumor cell group culture...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com