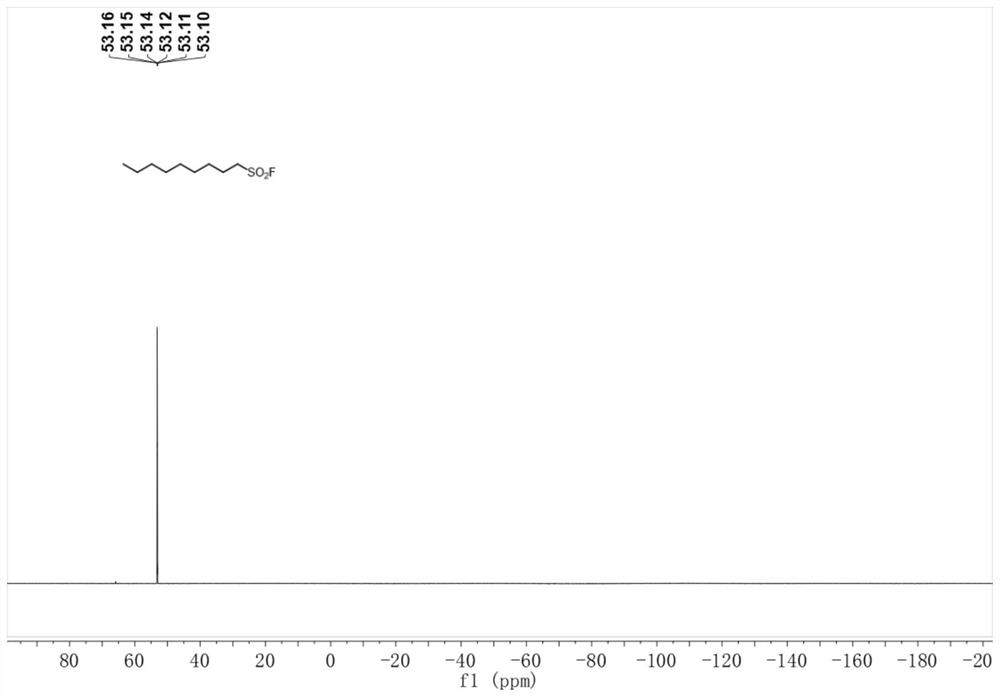
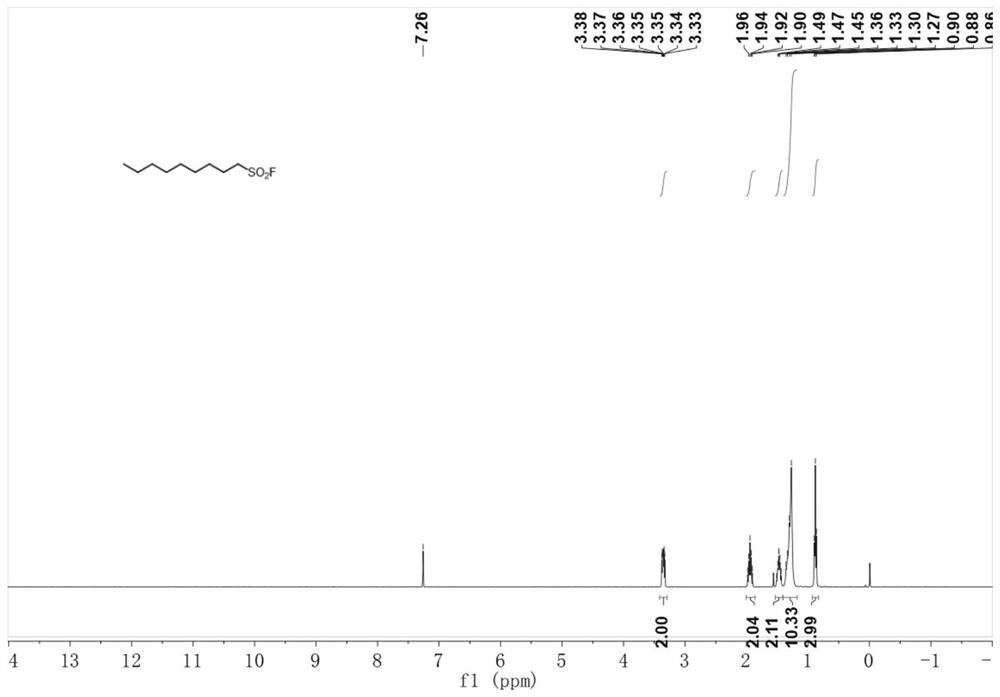
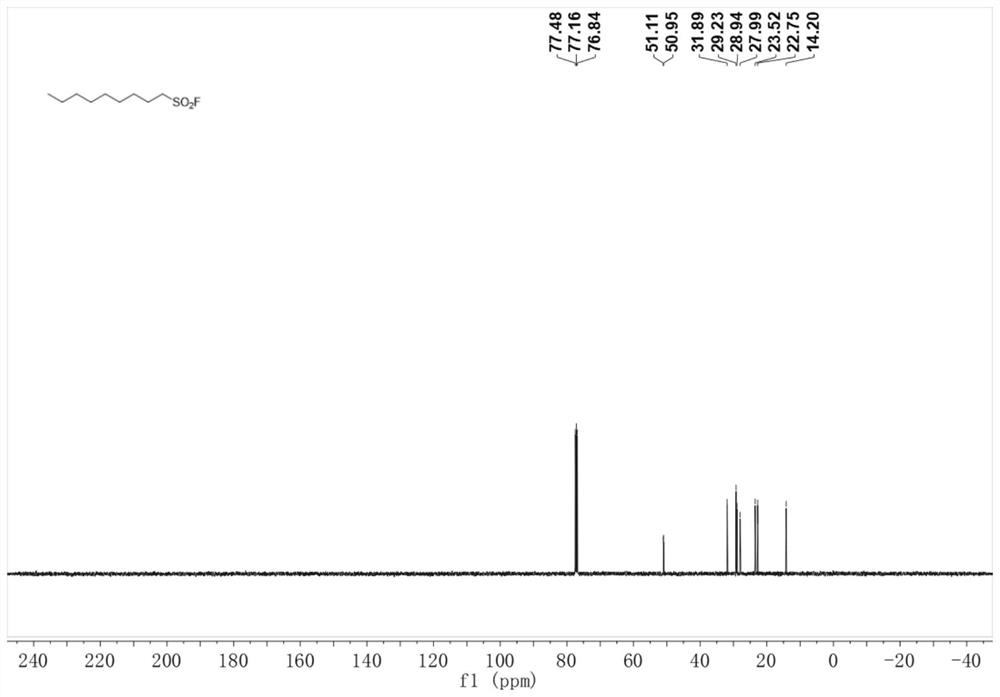
Method for preparing alkyl sulfonyl fluoride
A technology of alkylsulfonyl and alkyl groups, applied in the field of preparing alkylsulfonyl fluoride, can solve the problem of few synthesis methods, and achieve the effect of simple synthesis method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The method of preparing reduction activated esters of the alkyl carboxylic acid of the present invention comprises the steps of:
[0053]
[0054] Taking R as a cyclohexyl group, 1.28 g (10 mmol) of cyclohexylhalic acid, 1.63 g (10 mmol) N-hydroxyphthalimide, 0.12 g (1 mmol) DMAP, add 100 ml of two Chloromethane is solvent and stirred vigorously. Then 1.7 ml (10 mmol) DIC was added, and the mixture was stirred at room temperature for 3-5 hours. The mixture was filtered through diatomite or silica gel, and then flushed with dichloromethane. The filtrate was collected and the solvent was removed under reduced pressure. Purification by column chromatography or purification method to give the desired carboxylic acid reducing active ester raw material.
[0055] All carboxylic acid resection active esters of the present invention are prepared according to this method.
Embodiment 1
[0057] Cyclohexylsulfonyl fluoride synthesis, reducing active ester of cyclohexylcarboxylic acid as a template substrate, NA 2 S 2 O 4 As the sulfur dioxide source, NFSI is a fluorine source, a solvent is DMF, and a sulfonyl fluoride is formed under an inert gas protection and heating. The reactive and specific implementation are as follows:
[0058]
[0059] Take 10ml of the tube into the magnet, weigh 54.9 mg (0.2 mmol) reductive active ester of cyclohexylcycarboxylic acid, 52.3 mg (0.3 mmol) NA 2 S 2 O 4 The addition of the argon was added three times, and N, N-dimethylformamide (DMF) 2 ml was added to 80 ° C for 9 hours under argon. 0.6 mmol) NFSI reaction for 4 hours. After completion of the reaction, 4-methoxy trifluoromethoxybenzene was added as the internal standard, and the nuclear magnetic analysis was 0%.
Embodiment 2
[0061] Cyclohexylsulfonyl fluoride synthesis, reducing active ester of cyclohexylcarboxylic acid as a template substrate, NA 2 S 2 O 4 As the sulfur dioxide source, NFSI is fluorine source, solvent is DMF, water as an additive, and the reaction is formed into sulfonyl fluoride. The reaction is as follows:
[0062]
[0063] Take 10ml of the tube into the magnet, weigh 54.9 mg (0.2 mmol) reductive active ester of cyclohexylcycarboxylic acid, 52.3 mg (0.3 mmol) NA 2 S 2 O 4 Add it, the argon is placed three times, with N, N-dimethylformamide (DMF) and water under argon (V DMF : V 水 = 2: 0.4, mL) was used as a solvent and additive, and the mixture was heated to 80 ° C for 9 hours, cooled to room temperature, and the reaction was reacted for 4 hours. After completion of the reaction, 4-methoxy trifluoromethoxybenzene was added as the internal standard, and the nuclear magnetic analysis was added to the flu fluoroscopy rate of 22%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com