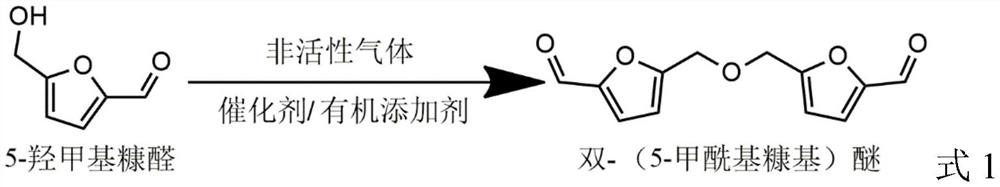
Preparation method of bis-(5-formyl furfuryl) ether
A formyl furfuryl, two-component technology, applied in the field of bis-ether preparation, to achieve the effect of mild reaction conditions, easy separation and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] 10mmol 5-hydroxymethylfurfural, 0.01mmol Cu(NO 3 ) 2 , 0.01 mmol VOSO 4 , 3mmol oxalic acid was added to a 50mL reaction kettle, the kettle was closed, and the air in the kettle was replaced with nitrogen gas 7 times, filled with 0.1MPa nitrogen gas, heated to 80°C, and reacted at this temperature for 0.5h. After the reaction, the reacted mixture was cooled to room temperature with water, acetonitrile was added to dissolve the solid, and then the catalyst was removed by filtration. The internal standard mesitylene was added, a sample was taken and analyzed by gas chromatography. Acetonitrile was removed by rotary evaporation, ethyl acetate was added and vacuum suction filtered, ethyl acetate was removed by rotary evaporation, and a solid was obtained by vacuum drying, with a gas chromatography (GC) purity of more than 99%.
[0060] The conversion rate of 5-hydroxymethylfurfural, the GC yield of bis-(5-formyl furfuryl) ether and the separation yield of bis-(5-formyl f...
Embodiment 2
[0062] 10mmol 5-hydroxymethylfurfural, 0.05mmol Ni(NO 3 ) 2 , 0.06mmol NaVO 3 , 2mmol of tartaric acid was added to a 50mL reaction kettle, the kettle was closed, and the air in the kettle was replaced with helium 7 times, filled with 5.0Mpa helium, heated to 50°C, and reacted at this temperature for 1h. After the reaction finished, according to the method described in Example 1, cooling and sampling analysis, the conversion rate of 5-hydroxymethylfurfural was 75%, and the GC yield of two-(5-formyl furfuryl) ether was 72%. The yield was 71%.
Embodiment 3
[0064] 10mmol 5-hydroxymethylfurfural, 0.1mmol Co(NO 3 ) 2 , 0.1 mmol VOPO 4 , 1mmol of acetylacetone was added to a 50mL reaction kettle, the kettle was closed, and the air in the kettle was replaced with nitrogen gas 7 times, filled with 3.0MPa nitrogen gas, heated to 60°C, and reacted at this temperature for 1.5h. After the reaction finished, according to the method described in Example 1, cooling and sampling analysis, the conversion rate of 5-hydroxymethylfurfural was 50%, and the GC yield of two-(5-formyl furfuryl) ether was 48%. The yield was 47%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com