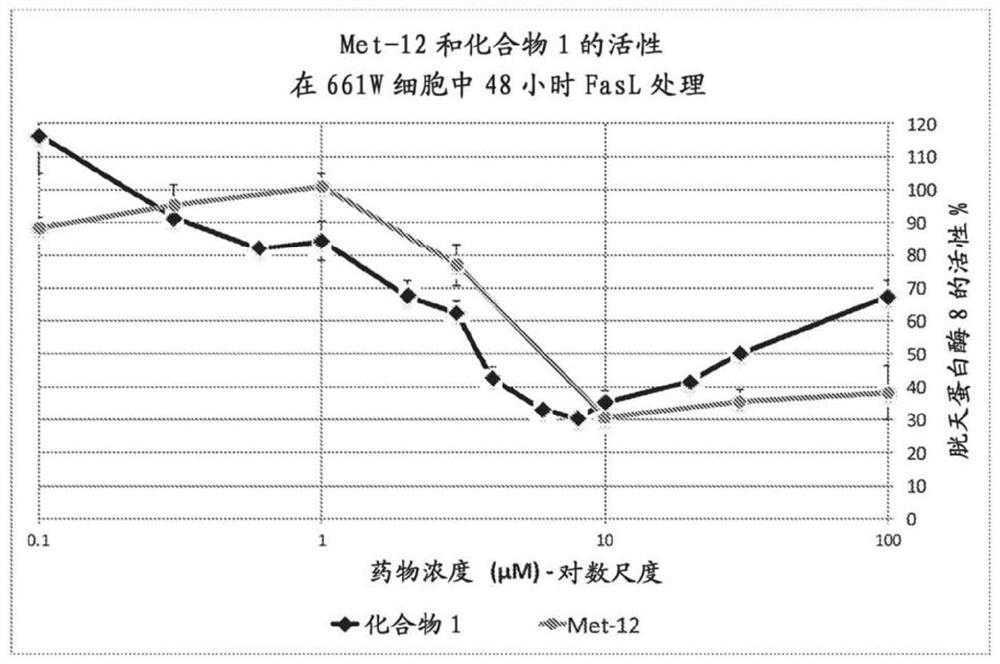
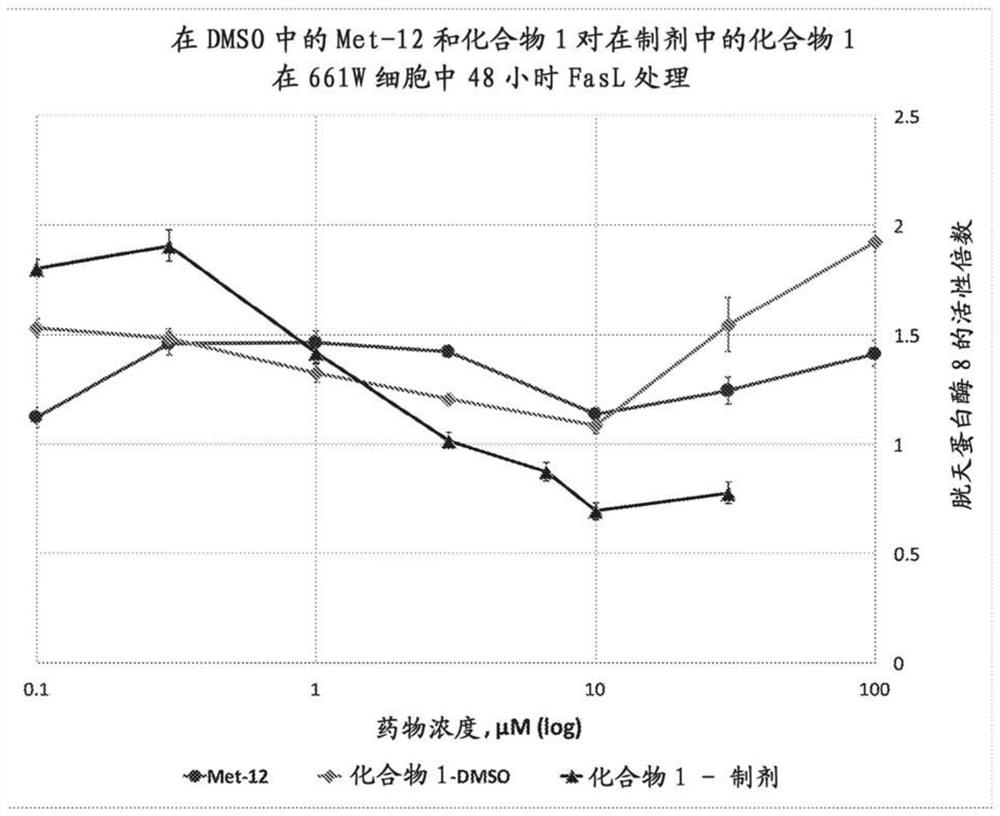
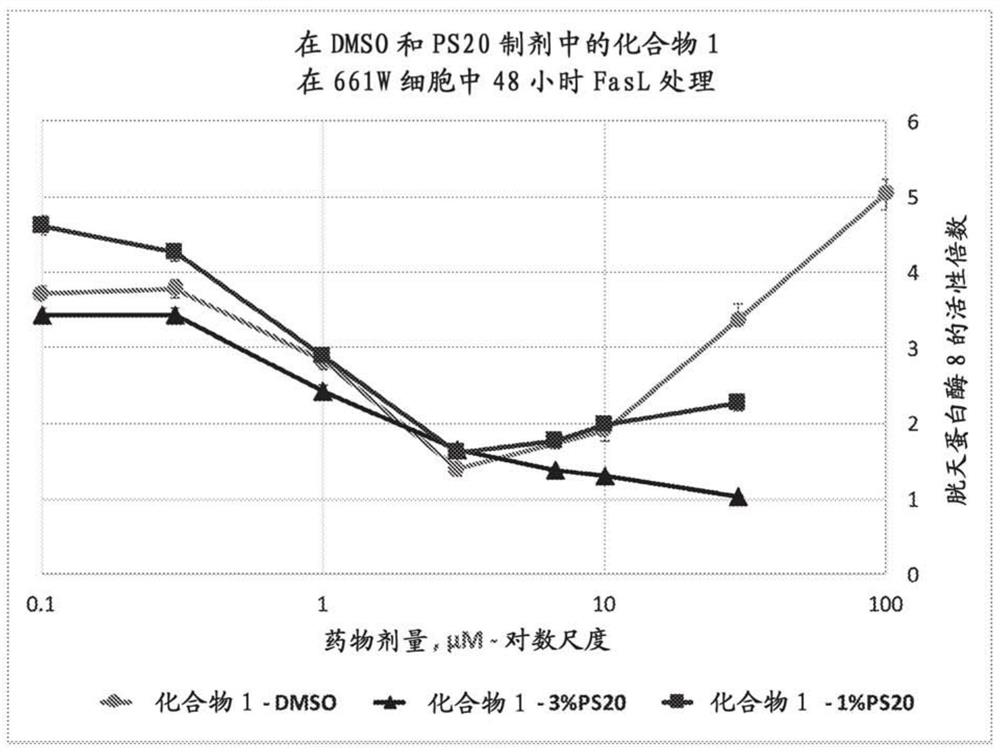
Peptide compositions and methods of use
A composition and compound technology, applied in the direction of botany equipment and methods, chemical instruments and methods, drug combination, etc., can solve the problems of large material loss, suboptimal, potency loss, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1: Compound 1 preparation and testing
[0110] Compound 1 peptide (peptide His-His-Ile-Tyr-Leu-Gly-Ala-Val-Asn-Tyr-Ile-Tyr- NH 2 ; SEQ ID NO: 1). Fmoc protected amino acids were purchased from GL Biochem. Reagents for coupling and cleavage were purchased from Aldrich. Solvents were purchased from Fisher Scientific.
[0111] The peptide chains are assembled on the resin by repeated removal of the Fmoc protecting group and coupling of the protected amino acids. DIC and HOBt were used as coupling reagents with NMM as the base; 20% piperidine in DMF was used as the de-Fmoc-reagent. A ninhydrin test was performed after each coupling to check coupling efficiency.
[0112] After the last coupling, the resin was washed and dried by cleavage mixture (TFA / Tis / H 2 O / DOTA: 95 / 3 / 2 / 2) to cleave the peptide from the resin. The peptide was precipitated from cold ether and collected by filtration to obtain 13 g of crude product with a purity of 46% (yield: 127%).
[011...
Embodiment 2
[0116] Example 2: pH-Solubility Curve of Compound 1
[0117] Compound 1 was obtained as the trihydrochloride salt as described in Example 1 and screened for water solubility at different pH by pH titration according to the following protocol. In some cases, Met-12 was run through the same experimental procedure to determine its pH solubility curve under the same conditions. Previous experiments have not identified any conditions where Met-12 can be satisfactorily formulated in substantially aqueous media at any pH above 2.7.
[0118] Compound 1 (10 mg) was dissolved in water (270-900 μL) in a 2 mL clear plastic centrifuge tube with vortexing, resulting in a solution with a pH of approximately 2.4. In all cases, the peptides formed clear solutions, demonstrating a solubility of at least 40 mg / mL at low pH. The solution was then diluted with an appropriate amount of co-solvent or other excipients (sugar\surfactant, etc.) to yield a clear acidic solution of 10 mg of Compound 1 ...
Embodiment 3
[0120] Example 3: pH-Dependent Solubility of Compound 1 in Cosolvent Mixtures
[0121] The pH-dependent solubility of compound 1 was examined using co-solvents and additives and compared with the solubility of Met-12 under the same conditions.
[0122] The 70% DMSO experiments were similar to the Met-12 titrations that formed gels around pH 5.5, but in this case, probably because the C-terminus could not be ionized, the gels were no longer soluble at higher pH values.
[0123] 70% propylene glycol (PG) improved the solubility of compound 1 compared to Met-12's pH 3.2, gelling did not occur until around pH 4.7, and then remained as a gel until pH 10. The titration was repeated with lower amounts of PG (35%, 10%), but no improvement in solubility over pure water seemed to be found.
[0124] The 70% PEG400 and 70% glycerol solutions did not seem to work, nor did the two sugar additives, 10% mannitol or 10% trehalose.
[0125] From these experiments it can be concluded that prop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com