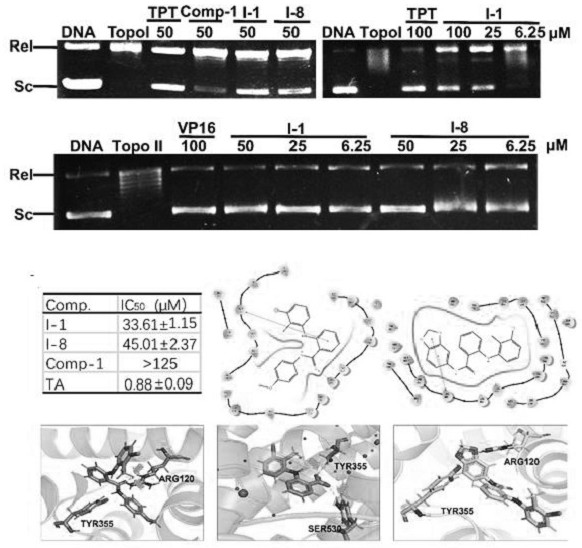
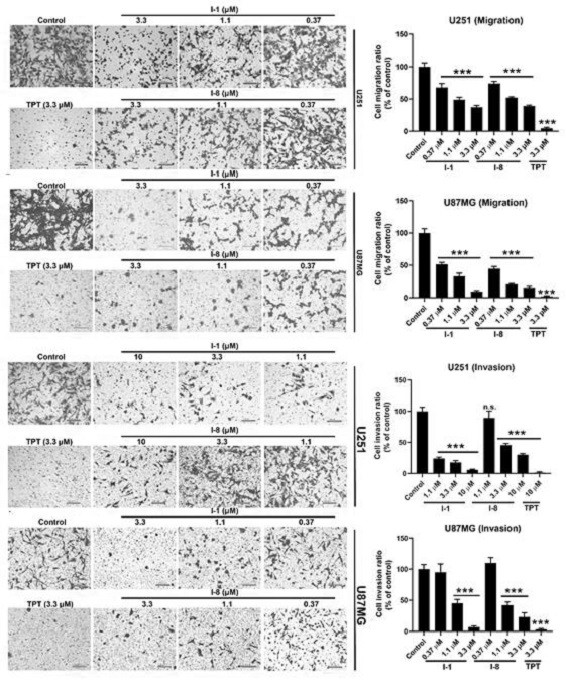
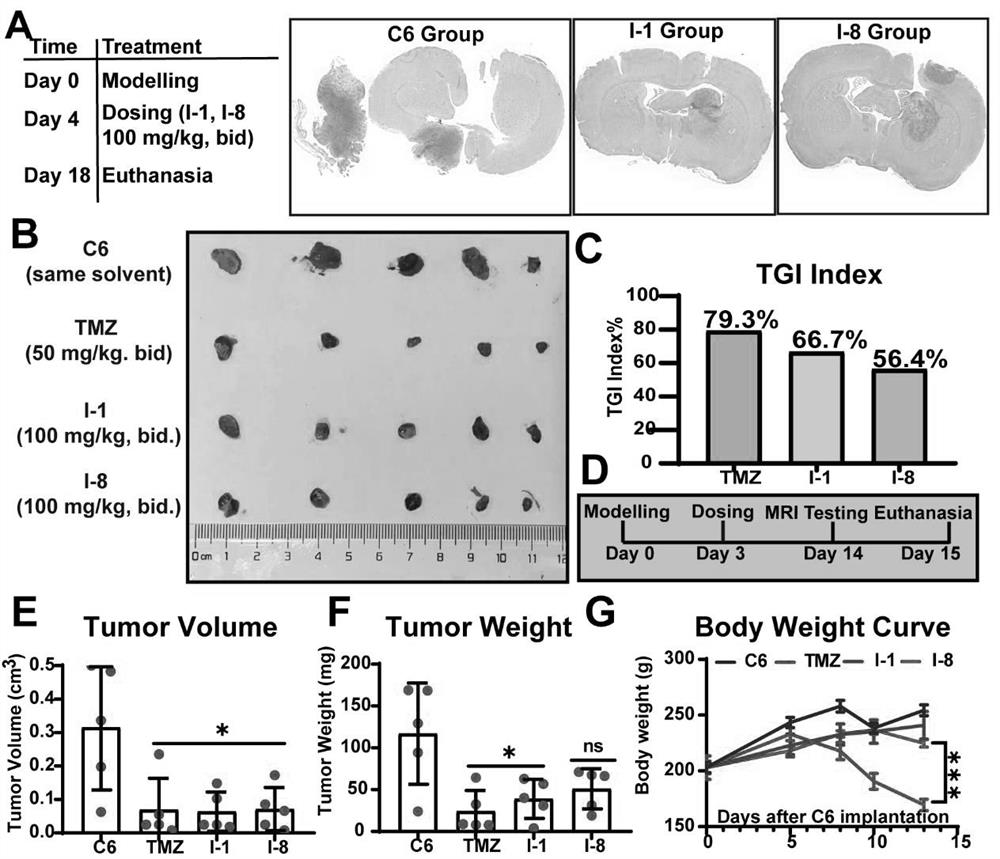
N-aryl anthranilamide compound, preparation and application thereof
An aminobenzamide and compound technology, which is applied to N-aryl anthranilamide compounds and the fields of preparation and application thereof, can solve the problems of not prolonging the median survival period, few species and the like, and achieves good resistance to various types of compounds. Human tumor activity, the effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of N-aryl anthranilamide compound, concrete steps are as follows:
[0035] Dissolve compound IV (1.0 eq), EDCI•HCl (1.2 eq), HOBT (1.1 eq) in dichloromethane (3 mL) under argon, and add Et 3 N (2.5 eq) and compound I (1.0 eq), stirred and reacted for 8 hours, after the completion of the reaction detected by TLC, the reaction solution was removed by rotary evaporation under reduced pressure, and then the obtained residue was purified by silica gel flash column chromatography to obtain the desired The solid product of the compound of formula (a), the total yield of the two steps is 20-50%.
Embodiment 2
[0037] A kind of N-aryl anthranilamide compound, concrete steps are as follows:
[0038] Dissolve compound IV (1.0 eq), EDCI•HCl (1.2 eq), HOBT (1.1 eq) in dichloromethane (3 mL) under argon, and add Et 3 N (2.5 eq) and compound II (1.0 eq), stirred and reacted for 12 hours, after the completion of the reaction detected by TLC, the reaction solution was removed by rotary evaporation under reduced pressure, and then the obtained residue was purified by silica gel flash column chromatography to obtain the desired The compound of formula (II) is a solid product, and the total yield of the two steps is 20-50%.
Embodiment 3
[0040] A kind of N-aryl anthranilamide compound, concrete steps are as follows:
[0041] Dissolve compound IV (1.0 eq), EDCI•HCl (1.2 eq), HOBT (1.1 eq) in dichloromethane (3 mL) under argon, and add Et 3 N (2.5 eq) and compound III (1.0 eq), stirred and reacted for 12 hours, after the completion of the reaction detected by TLC, the reaction solution was removed by rotary evaporation under reduced pressure, and then the obtained residue was purified by silica gel flash column chromatography to obtain the desired The compound of formula (III) is a solid product, and the total yield of the two steps is 20-50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com