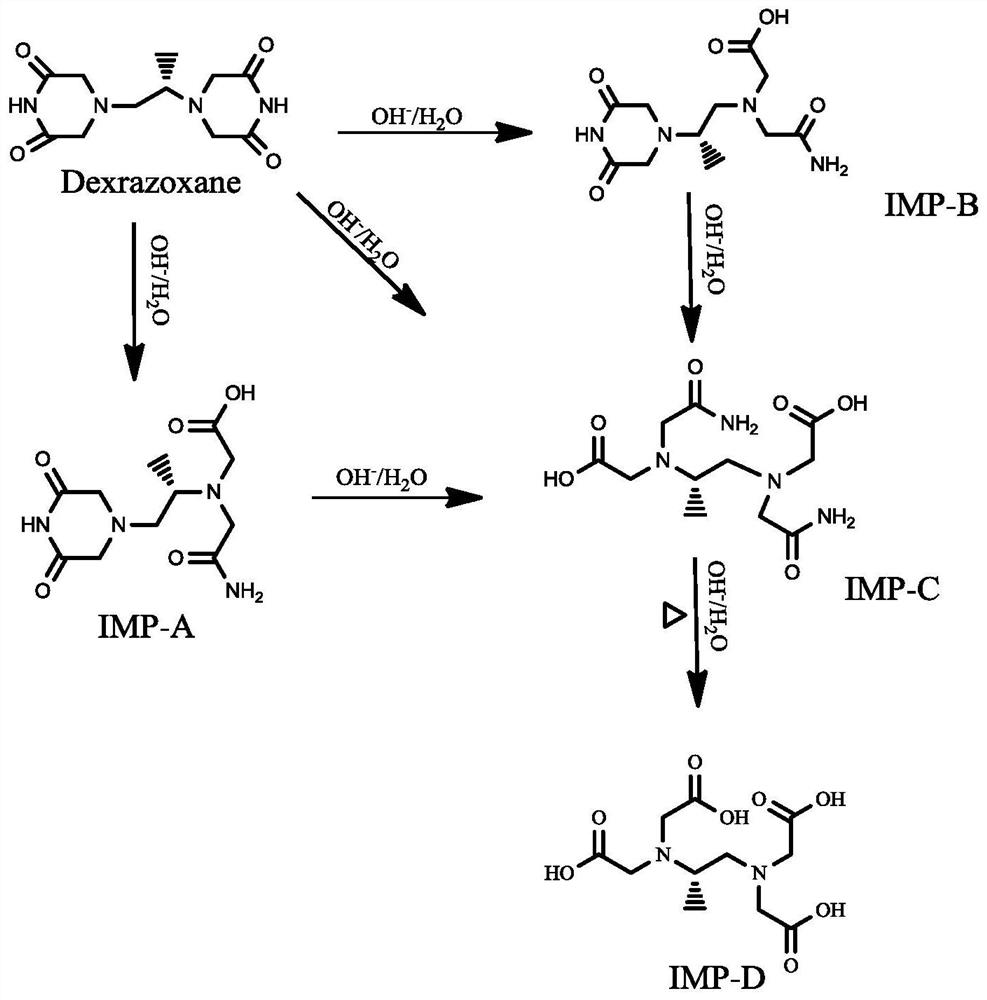
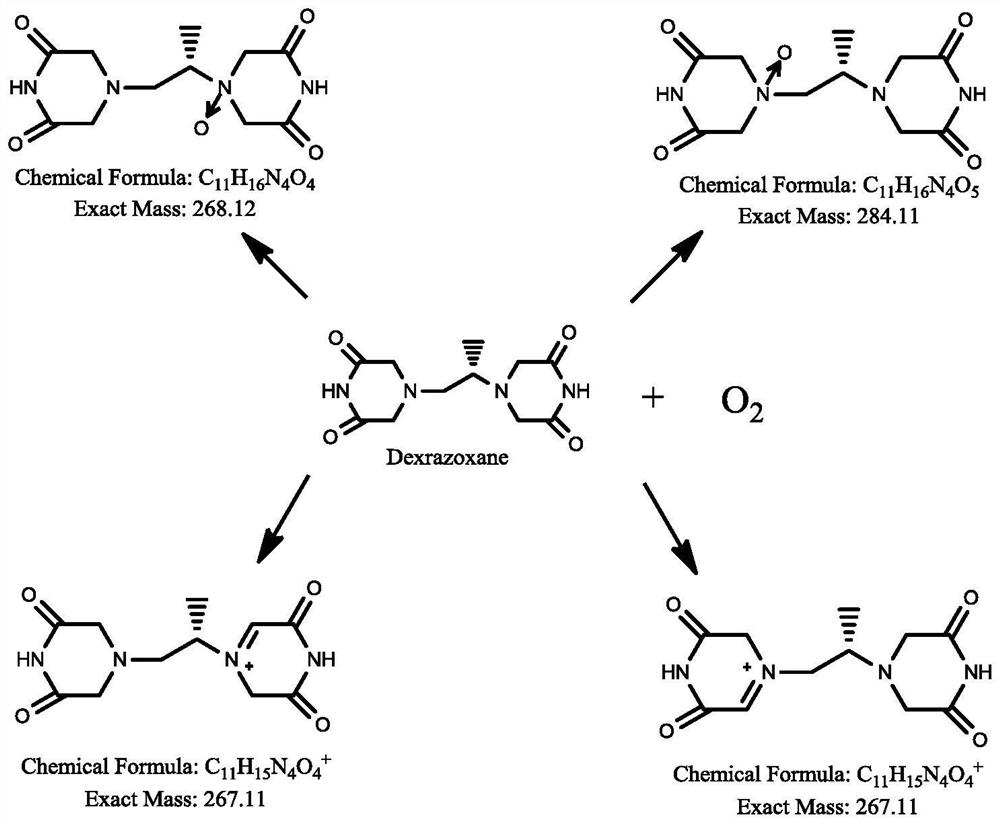
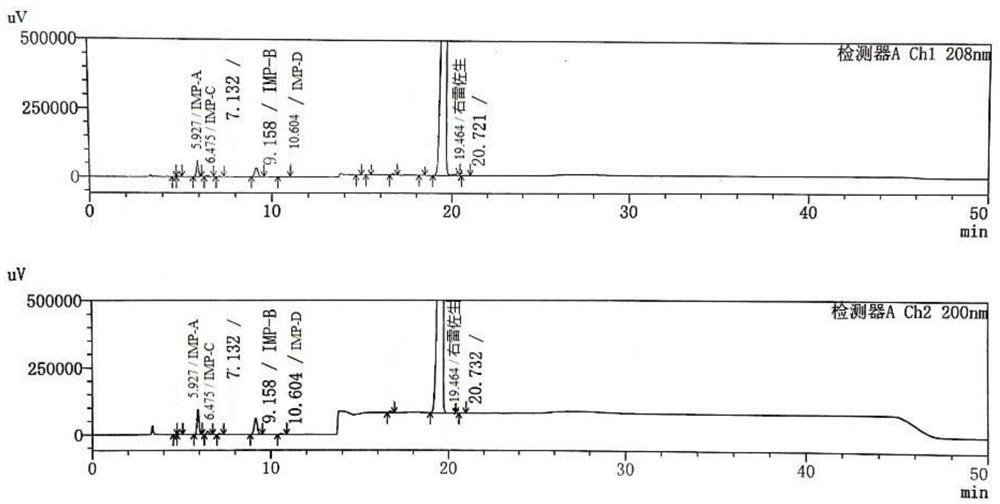
Dexrazoxane related substances and detection method thereof
A detection method and a technology for related substances, which are applied in the detection field, can solve the problems such as the lack of quality detection standards for dexrazoxane preparations, the inability to control the quality, and the lack of species, and achieve good system applicability and specificity, good linearity, and accuracy. Good effect of degree and durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] The screening of embodiment 1 chromatographic conditions
[0102] Screening of organic phase and elution procedure:
[0103] Condition 1:
[0104] Chromatographic column: YMC-Triart-C18, 4.6×250mm, 5μm;
[0105] Mobile phase: Phase A: phosphate buffer (0.01mol / L sodium dihydrogen phosphate aqueous solution, adjust pH to 2.2±0.05 with phosphoric acid), phase B: methanol, gradient elution program:
[0106] table 5
[0107] time / min Mobile phase A / vol.% Mobile phase B / vol.% 0 100 0 8.0 100 0 8.1 90 10 40.0 90 10 41.0 100 0 50.0 100 0
[0108] Column temperature: 30°C
[0109] Detection wavelength: 208nm;
[0110] Injection volume: 10μL;
[0111] Sample temperature: 5°C;
[0112] Diluent: Phosphate buffer (0.01mol / L sodium dihydrogen phosphate aqueous solution is adjusted to pH 1.5 with phosphoric acid, cooled to 2-8°C for use);
[0113] Test solution: resolution solution with a concentration of 1 mg / mL (prepared...
Embodiment 2
[0170] Embodiment 2 forced degradation test analysis
[0171] In order to verify that the detection method of the present invention can be applied to the quality control of dexrazoxane at various stages, the sample of dexrazoxane for injection is subjected to a forced degradation test, and the experimental method is as follows:
[0172] Acid destruction: Weigh about 55 mg of dexrazoxane sample for injection, put it in a 10 ml measuring bottle, add 4 ml of 1M hydrochloric acid aqueous solution, and place it at room temperature for 24 hours;
[0173] Alkali destruction: Weigh about 55 mg of dexrazoxane sample for injection, put it in a 10 ml measuring bottle, add 3 ml of 0.1M NaOH aqueous solution, and place it at room temperature for 10 minutes;
[0174] Oxidative damage: weigh about 55 mg of dexrazoxane for injection, put it in a 10 ml measuring bottle, add 1.5 ml of 0.3% hydrogen peroxide solution, and place it at room temperature for 1.5 hours;
[0175] High-temperature des...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com