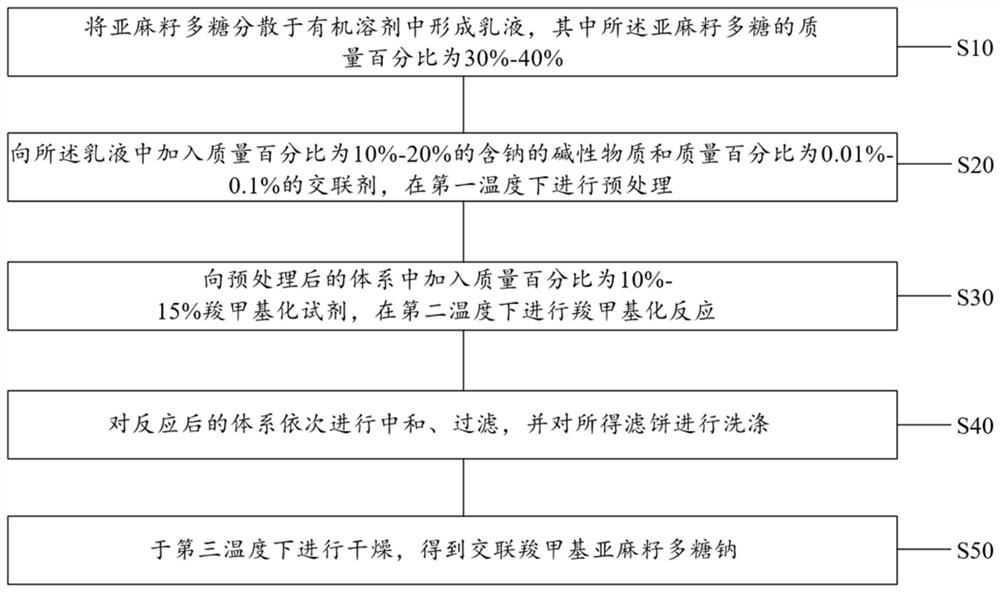
American ginseng orally disintegrating tablet as well as preparation method and application thereof
A technology of American ginseng and orally disintegrating tablets, which is applied in applications, medical formulas, and medical preparations of non-active ingredients, etc., can solve problems such as difficulty in swallowing tablets and capsules, and affect compliance with drug treatment, so as to enhance cellular immunity, The effect of reducing oral and throat mucosal reaction and promoting wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
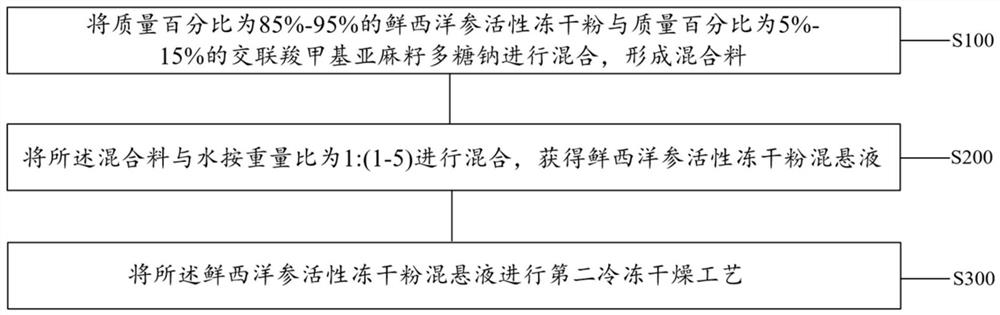
[0037] refer to figure 2 , the embodiment of the present invention also provides a preparation method of American ginseng orally disintegrating tablets, comprising:
[0038] Step S100: mixing 85%-95% by mass of active freeze-dried fresh American ginseng powder with 5%-15% by mass of cross-linked carboxymethyl linseed polysaccharide sodium to form a mixture;
[0039] Step S200: mixing the mixture with water in a weight ratio of 1:(1-5) to obtain a suspension of fresh American ginseng active freeze-dried powder;
[0040] Step S300: The fresh American ginseng active freeze-dried powder suspension is subjected to a second freeze-drying process.
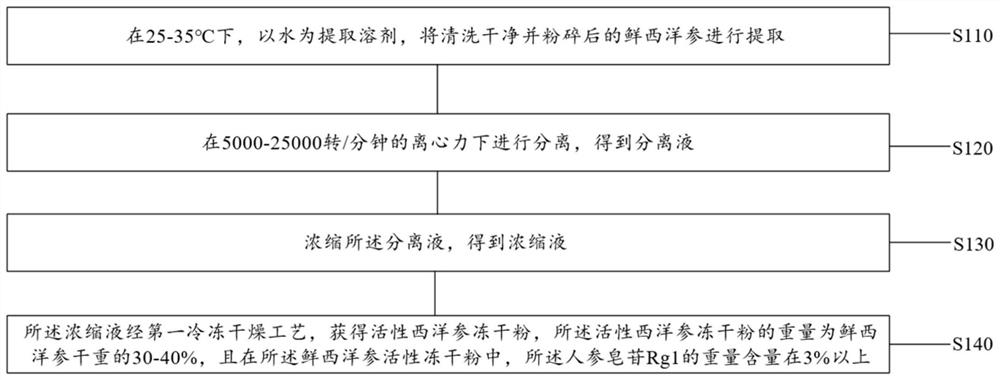
[0041] refer to image 3 , in step S100, the preparation method of the active freeze-dried powder of fresh American ginseng comprises:
[0042] Step S110: Extracting the washed and crushed fresh American ginseng at 25-35°C with water as the extraction solvent;
[0043] Step S120: Separating under a centrifugal force of 5000-25000 rpm...
Embodiment 1
[0077] (1) Preparation of fresh American ginseng active freeze-dried powder: take 10 kg of fresh American ginseng, clean it with distilled water, grind it into 60-100 mesh particles, and extract it using continuous countercurrent ultrasonic extraction equipment at 25-35 ° C. The solvent is extracted, and the extract is centrifuged and then concentrated by a reverse osmosis concentrator (Hefei Zhixuan Membrane Separation Technology Co., Ltd.) to obtain a concentrate and reverse osmosis water. Reverse osmosis water is recycled as extraction solvent to realize green production. The concentrated solution is freeze-dried to obtain 1.02 kg of active freeze-dried powder of fresh American ginseng. In the active freeze-dried fresh American ginseng powder, the weight content of ginsenoside Rg1 is 3.15%, and the extraction rate of American ginseng saponin is 99.0%.
[0078] (2) Preparation of cross-linked carboxymethyl linseed polysaccharide sodium: add 2500g linseed polysaccharide to 5...
Embodiment 2
[0082] Refer to Example 1 for the preparation method of fresh American ginseng active freeze-dried powder and cross-linked carboxymethyl linseed polysaccharide sodium.
[0083]Preparation of American ginseng orally disintegrating tablets: Take 500 grams of fresh American ginseng active freeze-dried powder, 40 grams of cross-linked carboxymethyl linseed polysaccharide sodium, fully mix the above materials in a three-dimensional mixer to obtain a mixture, and add 550 grams of distilled water to the mixture , mixed to make a suspension, the suspension was quantitatively added to the prefabricated double-aluminum blister, and after quick-freezing in a liquid nitrogen tunnel, freeze-drying (pre-freezing stage: the product is put into the box, the product is cooled to -65 ° C, and the -65°C and keep it for 1 hour, the pre-freezing stage is over; sublimation stage: the temperature of the plate layer is controlled at 10°C, and the temperature of the product is maintained at -20°C until...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com