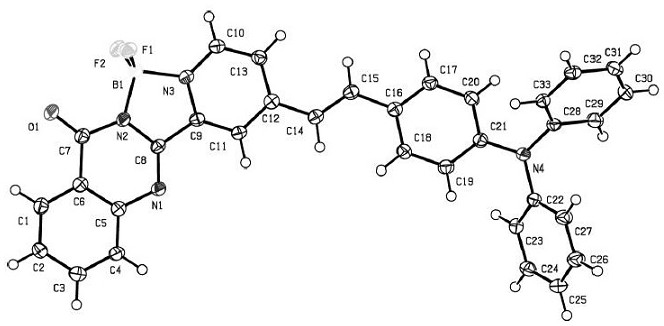
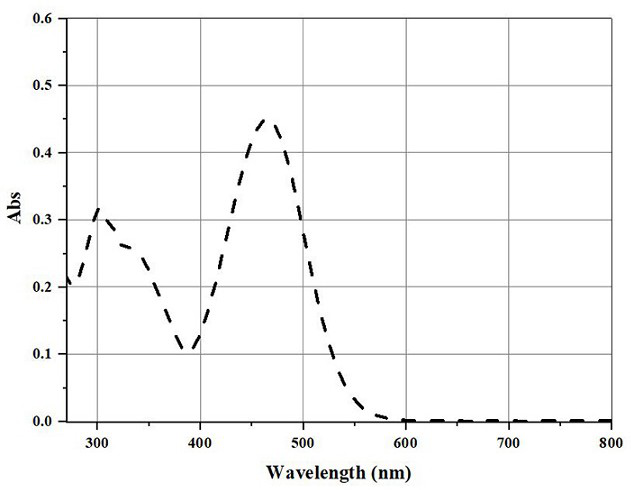
A dual function probe for endoplasmic reticulum localization imaging/light-induced ferroptosis
A light-induced, endoplasmic reticulum technology, used in fluorescence/phosphorescence, microbial assay/inspection, luminescent materials, etc., can solve the problem of lack of good monitoring methods, compounds without organelle targeting, and difficulty in direct observation of lipid peroxidation changes. wait until the problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051]
[0052] In a 100mL reactor, add (E)-2-(4-(4-(diphenylamino)styryl)pyridin-2-yl)quinazolin-4(3H)-one 4.92g (10mmol) , add 50 mL of toluene as a solvent, stir and mix evenly, add 1.38 mL (10 mmol) of triethylamine and boron trifluoride·diethyl ether complex, heat under reflux, react for 30 h, pour the reaction solution into water after the reaction, wait for After separation, extraction was performed with dichloromethane, and the organic phase was concentrated using a rotary evaporator to remove the solvent. The target product is obtained by separation by column chromatography. The yield was 45%. The following are the NMR and MS experimental data of the product:
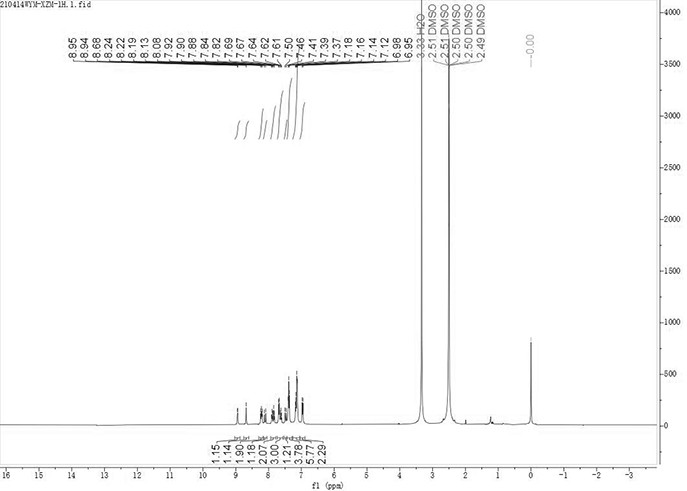
[0053] 1 H NMR (400 MHz, DMSO-d 6 ) δ =8.94 (d, J = 5.9 Hz, 1H), 8.68 (s, 1H), 8.30 – 8.16 (m, 2H), 8.11 (d, J = 16.3 Hz, 1H), 7.94 – 7.78 (m, 2H) ), 7.66(dd, J = 19.8, 7.8 Hz, 3H), 7.48 (d, J = 16.3 Hz, 1H), 7.39 (t, J = 7.7 Hz, 4H), 7.15 (dd, J = 17.6, 7.5 Hz , 6H), 6.96 (d, J = 8.5 Hz, 2H) ppm.
[...
Embodiment 2
[0074]
[0075] In a 100 mL reactor, add (E)-6-(dimethylamino)-2-(4-(4-(diphenylamino)styryl)pyridin-2-yl)quinazoline-4( 3H)-ketone 5.35g (10mmol), add 50mL of toluene as a solvent, stir and mix well, add triethylamine 1.38mL (10mmol) and boron trifluoride·diethyl ether complex, heat under reflux, react for 30h, the reaction ends Then, the reaction solution was poured into water, and after the layers were separated, the mixture was extracted with dichloromethane, and the organic phase was concentrated using a rotary evaporator to remove the solvent. The target product is obtained by separation by column chromatography. The yield was 45%. The following are the NMR and MS experimental data of the product:
[0076] 1 H NMR (400 MHz, Chloroform-d) δ= 9.00 (d, J = 8.5 Hz, 1H), 8.56 (d, J = 1.8 Hz, 1H), 7.86 (s, 1H), 7.52 (d, J = 2.3 Hz, 1H), 7.46 (d, J = 8.1 Hz, 1H), 7.45 – 7.39 (m, 2H), 7.39 – 7.34 (m, 1H), 7.32 – 7.25 (m, 4H), 7.29 –7.19 (m, 1H), 7.17 – 7.08 (m, 7H), 7.04...
Embodiment 3
[0086]
[0087] In a 100 mL reactor, add (E)-2-(4-(4-(diphenylamino)styryl)pyridin-2-yl)benzo[g]quinazolin-4(3H)-one 5.43 g (10 mmol), 50 mL of toluene was added as a solvent, after stirring and mixing uniformly, 1.38 mL (10 mmol) of triethylamine and boron trifluoride·diethyl ether complex were added, and the mixture was heated to reflux for 30 h. Pour into water, wait for separation, extract with dichloromethane, and use a rotary evaporator to concentrate the organic phase to remove the solvent. The target product is obtained by separation by column chromatography. The yield was 45%. The following are the NMR and MS experimental data of the product:
[0088] 1 H NMR (400 MHz, Chloroform-d) δ= 9.00 (d, J = 8.5 Hz, 1H), 8.57 (d, J = 1.9 Hz, 1H), 8.50 (dd, J = 2.0, 0.7 Hz, 1H), 8.27 (d, J = 1.9 Hz, 1H), 7.94 (ddd, J = 7.8, 2.0, 1.2 Hz, 1H), 7.85 (s, J = 8.4 Hz, 1H), 7.61 – 7.47(m, 2H), 7.46 – 7.39 (m, 2H), 7.39 – 7.33 (m, 1H), 7.33 – 7.24 (m, 4H), 7.29 – 7.18 (m, 1H), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com