Fluorescent dye probe for latent fingerprint detection as well as preparation method and application of fluorescent dye probe
A fluorescent dye and latent fingerprint technology, applied in applications, luminescent materials, chemical instruments and methods, etc., can solve the problems of high toxicity, reagents cannot be stored for a long time, and latent fingerprint imaging difficulties, etc., to achieve low toxicity and ensure biological safety and low toxicity, avoiding the effect of repeated washing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
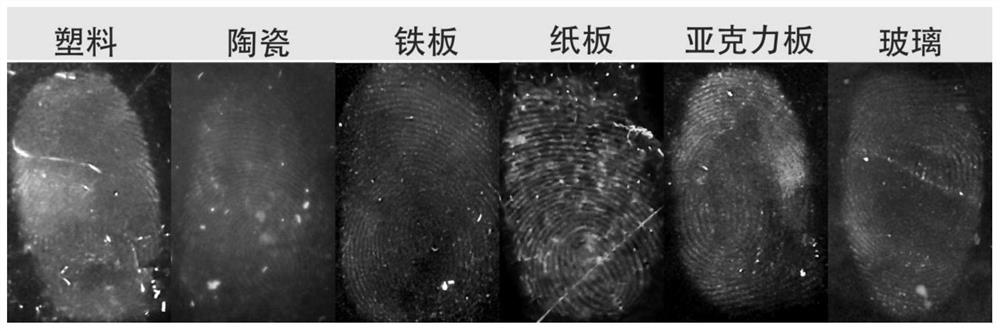
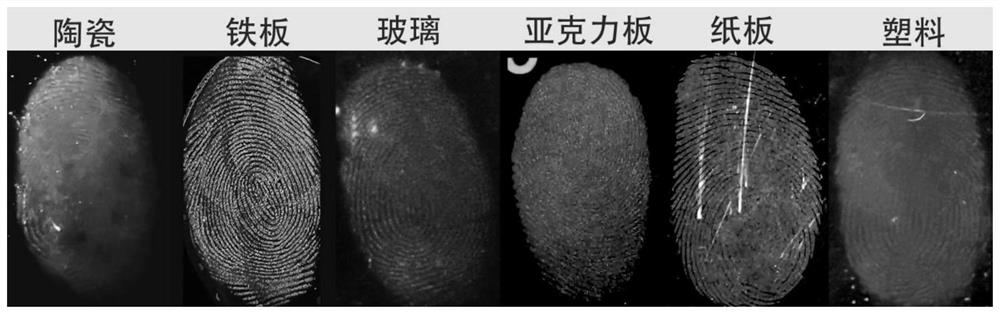
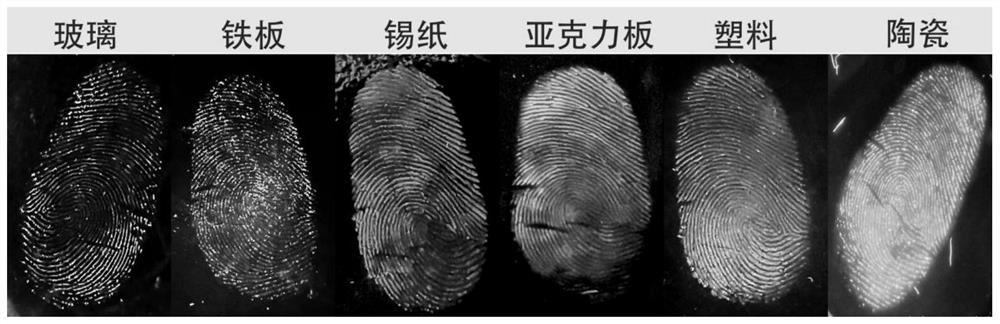
Image
Examples
preparation example Construction
[0050] As mentioned above, the preparation method of fluorescent dye probes for latent fingerprint detection, the overall method is similar to FLYSTracker@Yellow and probe FLYSTracker@Yellow in this example, and when the same or similar method is used for fingerprint imaging, it can also be obtained Better fingerprint imaging effect, the method comprises the following steps:
[0051] (1) In the reactor, add N-acetylglycine, R 3 Aldehyde, sodium acetate and acetic anhydride, reflux reaction under the protection of nitrogen, after the reaction, cool until there is solid precipitation, then wash off the anhydride, filter, then wash, and dry to obtain the product A; N-acetylglycine and R 3 The mol ratio of aldehyde is (1.1-1.3): 1, the time of reflux reaction is 2-10h, and the reaction formula is as follows:
[0052]
[0053] (2) In the reactor, add compound A and R respectively 2 amine, and then add a solvent to submerge the solid, reflux reaction overnight under the protect...
Embodiment 1
[0065] Synthesis of fluorescent dye probe FLYSTracker@Yellow:
[0066] (1) Preparation of Compound 1:
[0067]
[0068] In a 100mL round bottom flask, add N-acetylglycine (500mg, 4.27mmol), p-dimethylaminobenzaldehyde (530mg, 3.56mmol) and sodium acetate (437mg, 5.33mmol), acetic anhydride (6ml) respectively, Under the protection of nitrogen, the reaction was refluxed for 3h. After cooling to room temperature, a solid precipitated out. The acid anhydride was washed away with 15 ml of n-hexane, filtered, washed with cold ethanol, and dried overnight at 60° C. in a vacuum desiccator to obtain the product as a dark red solid with a yield of 65%. 1 H NMR (400MHz, CDCl 3 ) 8.00 (d, J=8.9Hz, 2H), 7.09 (s, 1H), 6.70 (d, J=9.0Hz, 2H), 3.09 (s, 6H), 2.37 (s, 3H).
[0069] (2) Preparation of compound 2:
[0070]
[0071] In a 100mL round-bottomed flask, were added compound 1 (500mg, 2.17mmol), N,N-dimethylethylenediamine (474μL, 4.34mmol), potassium carbonate (495mg, 3.25mmol)...
Embodiment 2
[0083] Synthesis of fluorescent dye probe FLYSTracker@Red:
[0084] (1) Preparation of compound 3:
[0085]
[0086] In a 100mL round bottom flask, add respectively compound 2 (500mg, 1.6mmol) in Example 1, p-dimethylaminobenzaldehyde (358mg, 2.4mmol), zinc chloride (2.2g, 16mmol), dioxygen Hexacyclic (20mL), reflux reaction under nitrogen protection for 2-4h. Cool and remove the solvent under reduced pressure. Column chromatography with dichloromethane gave a purple solid with a yield of 60%. 1 HNMR (400MHz, CDCl 3 )δ9.73(s,1H),7.99(d,J=8.8Hz,2H),7.73(d,J=8.8Hz,2H),7.08(s,1H),6.71(s,5H),3.07( s,18H),2.36(s,4H).
[0087] (2) Preparation of probe FLYSTracker@Red fluorescent dye probe for latent fingerprint imaging:
[0088]
[0089] Compound 3 (100mg, 0.23mmol) was dissolved in 80ml of dichloromethane and heated to dissolve completely, then filtered while hot. Add iodomethane (0.57ml, 6.26mmol) to the filtrate and stir at room temperature for 48 hours. Precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com