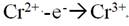Purification method of iron-chromium electrolyte and iron-chromium electrolyte obtained therefrom
A purification method and electrolyte technology, which is applied in the field of iron-chromium electrolyte and purified iron-chromium electrolyte, can solve problems such as electrolyte failure, hydrogen evolution, occurrence, etc., and achieve the goals of avoiding harmful side reactions, ensuring purity, and reducing costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
[0043]In the first embodiment of the present invention, a method for purifying an iron-chromium electrolyte for a flow battery is provided, which is characterized in that it includes the following steps:
[0044] Steps for providing an electrolytic cell having an anode, an anolyte, a cathode, and a membrane:
[0045] The step of passing the iron-chromium electrolyte to be purified through the surface of the cathode;
[0046] Wherein, the cathode includes metallic mercury and a porous conductive material, and when the iron-chromium electrolyte to be purified passes through the surface of the cathode, a reduction reaction occurs at least in part of the surface area of the cathode under the action of an external current.
[0047] anode and anolyte
[0048] In an embodiment of the invention, the anode comprises an anode material. The anode material may include a carbon-based material having a porous structure, and the pores can form a communication structure that accommodate...
no. 2 approach
[0079] The second embodiment of the present invention relates to a purification device for iron-chromium electrolyte, comprising the following structure:
[0080] Cell;
[0081] Cathode material import / export,
[0082] Wherein, the electrolytic cell includes an anode, a cathode and a diaphragm,
[0083] the cathode comprises metallic mercury and a porous conductive material, and
[0084] A space is formed between the cathode surface and the separator, which space is able to accommodate or allow the passage of the iron-chromium electrolyte to be purified.
[0085] The electrolytic cell is divided by a diaphragm into an anode part and a cathode part, and the anode part includes an anode material which is the same as that in the first embodiment of the present invention.
[0086] The cathode portion includes a cathode and a space formed between a separator and the cathode. The separator and cathode are the same as described in the first embodiment of the present invention. The...
Embodiment 1
[0112] In addition to introducing graphite felt, the electrode surface area of the cathode is increased to about 1100 cm 2 1. Except that the flow velocity of the iron-chromium electrolyte becomes 1L / min, the iron-chromium negative electrode electrolyte after purification in Comparative Example 1 is further purified using the same purification device and method as in Comparative Example 1.
[0113] After further purification, the impurity contents of various metal ions in the iron-chromium electrolyte are as follows: Cu content is 10ppb, Ni content is 15ppb, Pt content is 3ppb, Au content is 0, Ag content is 8ppb, and the total content is 36ppb.
[0114] After the iron-chromium electrolyte is purified by purification equipment, it is loaded with a conventional reaction area of 25cm 2 , the positive and negative liquid storage bottles are respectively 150ml liquid flow cells for charge and discharge cycle.
[0115] The battery is continuously charged and discharged, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com