NIR response type biomimetic membrane nano vesicle as well as construction method and application thereof
A technology of nanovesicles and construction methods, which can be applied to pharmaceutical formulations, preparations for in vivo experiments, medical preparations with non-active ingredients, etc., and can solve problems such as T cell exhaustion and the inability to exert cytotoxic T cell immune response effects , to inhibit the growth of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
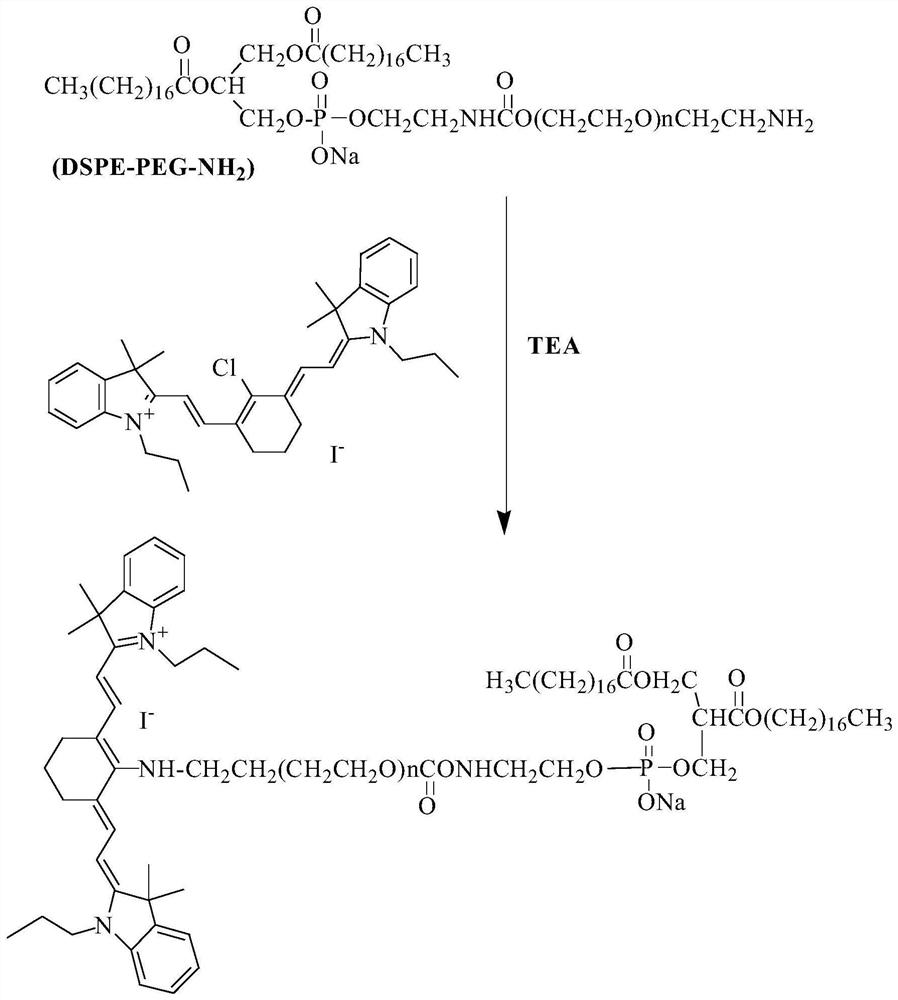
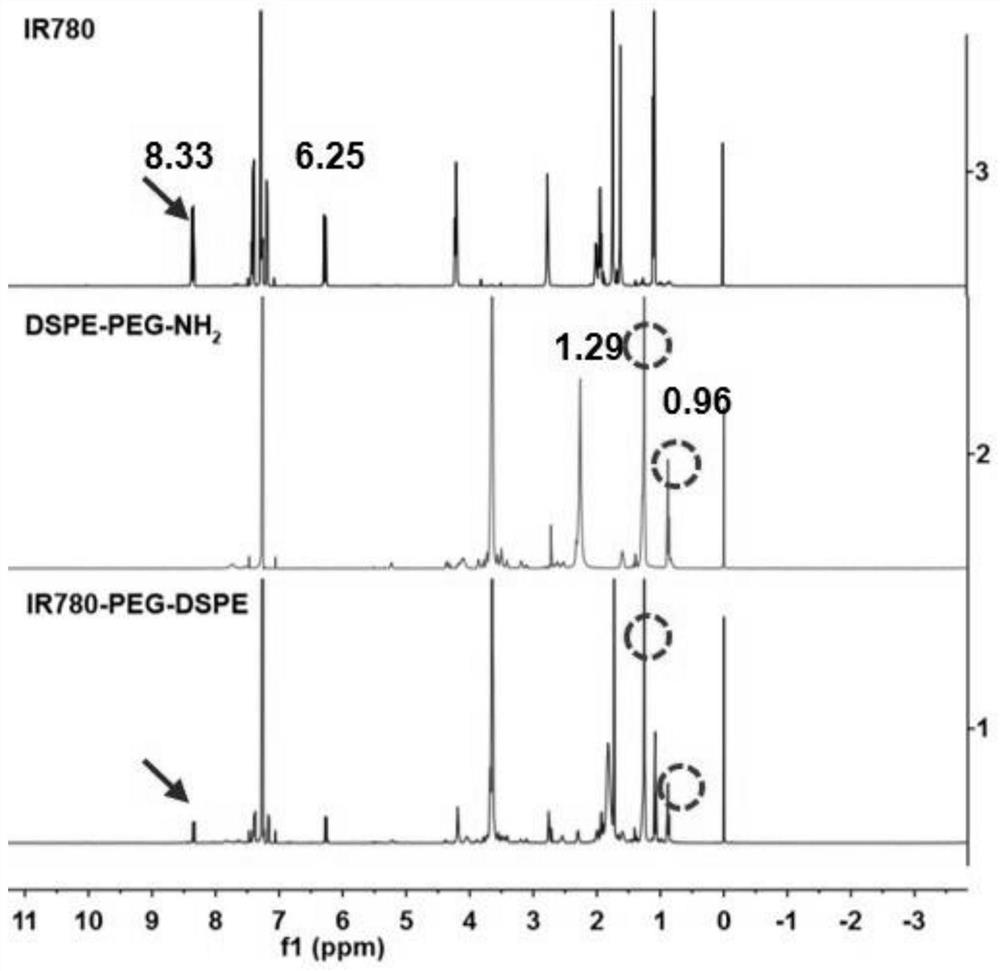
[0079] 1) Preparation of an IR-780 iodide-modified lipid graft (IR780-PEG2000-DSPE)
[0080] The IR-780 iodide-modified lipid graft (IR780-PEG2000-DSPE) provided in Example 1 is synthesized by a substitution method, which specifically includes the following steps:
[0081] Weigh 10mg of IR-780 iodide and 40mg of NH 2 -PEG2000-DSPE, accurately weighed, dissolved in dichloromethane solution to obtain a dichloromethane solution containing IR-780 iodide; with triethylamine (TEA) as the acid-binding agent, triethylamine (TEA) and The molar ratio of IR-780 iodide is 2: 1 ratio and weighs triethylamine (TEA), and adds in the described dichloromethane solution that contains IR-780 iodide, room temperature stirring reaction 24h; After the reaction finishes, Add the reaction solution to 10 times the volume of anhydrous ether, overnight at 4°C, then centrifuge at 3000 rpm for 5 minutes to remove by-products, and then dry the precipitate to obtain IR-780 iodide-modified lipids Graft (IR...
Embodiment 2
[0094] 1) Preparation of an IR-780 iodide-modified lipid graft (IR780-PEG2000-DSPE)
[0095] The preparation method of the IR-780 iodide-modified lipid graft (IR780-PEG-2000-DSPE) provided in Example 2 is the same as that in Example 1, and no further description is made here.
[0096] 2) About the construction of a thermosensitive lipid drug delivery system
[0097] The thermosensitive lipid drug delivery system provided in Example 2 is constructed through the following steps:
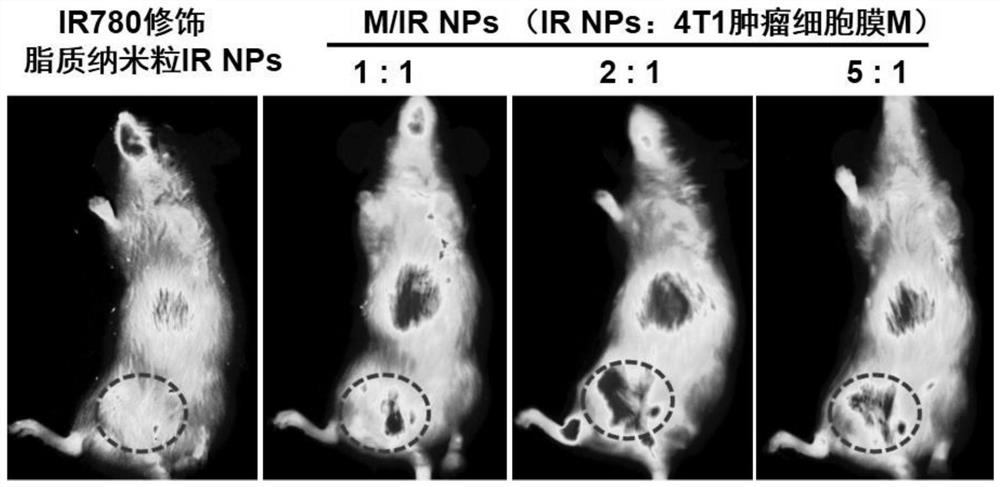
[0098] Taking the inhibitor BMS202 (BMS) as a model drug, weigh the IR-780 iodide-modified lipid graft (IR780-PEG2000-DSPE) obtained in the inhibitor BMS202, Example 1, thermosensitive lipid DPPC and cholesterol respectively, and Dissolve the weighed inhibitor BMS202, IR-780 iodide-modified lipid graft (IR780-PEG2000-DSPE), thermosensitive lipid DPPC and cholesterol in ethanol, and heat in a water bath at 60°C to obtain the ethanol phase Solution (in this solution, the mass percentage of inhibitor BM...
Embodiment 3
[0105] 1) Preparation of an IR-780 iodide-modified lipid graft (IR780-PEG2000-DSPE)
[0106] The preparation method of the IR-780 iodide-modified lipid graft (IR780-PEG2000-DSPE) provided in Example 3 is the same as that in Example 1 and will not be described here.
[0107] 2) About the construction of a thermosensitive lipid drug delivery system
[0108] The thermosensitive lipid drug delivery system provided in Example 3 is prepared through the following steps:
[0109] Taking the inhibitor BMS202 (BMS) as a model drug, weigh the IR-780 iodide-modified lipid graft (IR780-PEG2000-DSPE) obtained in the inhibitor BMS202, Example 1, thermosensitive lipid DPPC and cholesterol respectively, and Dissolve the weighed inhibitor BMS202, IR-780 iodide-modified lipid graft (IR780-PEG2000-DSPE), thermosensitive lipid DPPC and cholesterol in ethanol, and heat in a water bath at 60°C to obtain the ethanol phase Solution (in this solution, the mass percentage of inhibitor BMS202 is 20%, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com