Polypeptide, pharmaceutical composition containing polypeptide and application of polypeptide and pharmaceutical composition
A composition and drug technology, applied in the field of polypeptides and pharmaceutical compositions containing the polypeptides and biological drugs, can solve the problems of prolonging half-life, imperfect drug delivery system, poor drug stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0214] Embodiment 1: the preparation of polypeptide
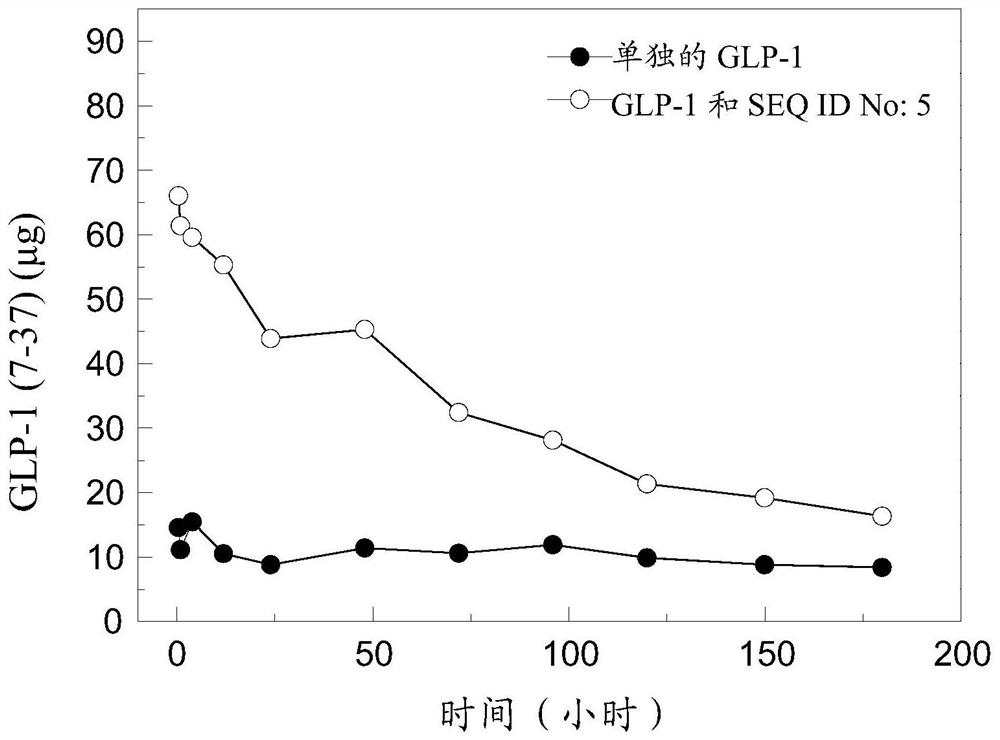
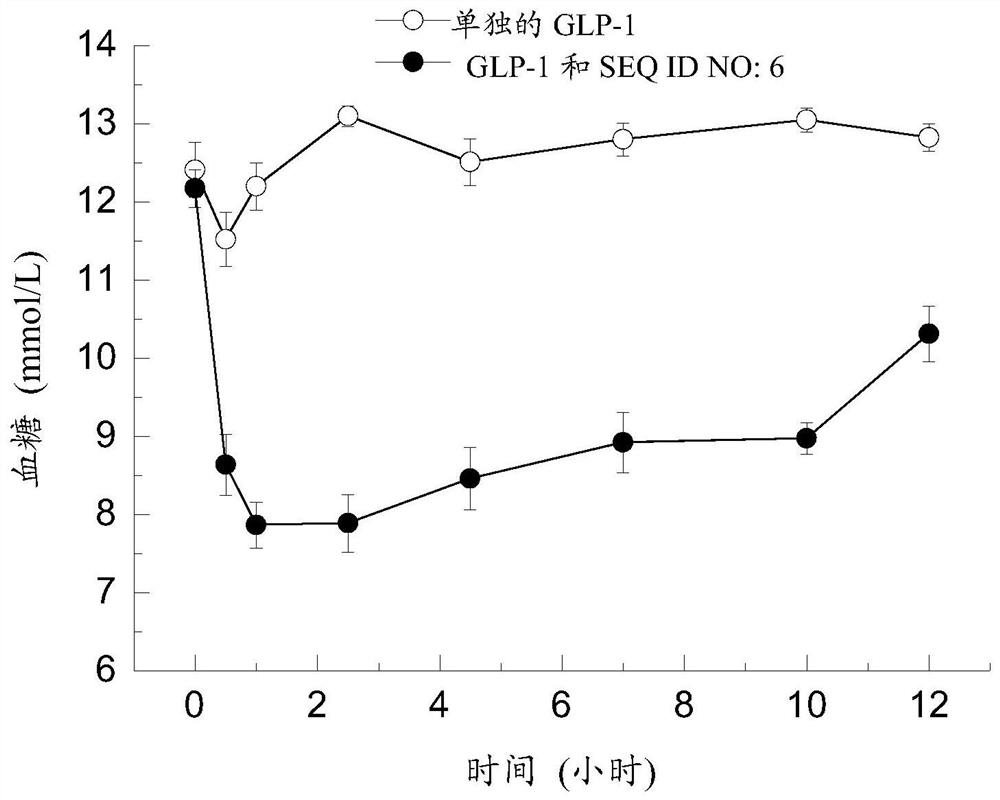
[0215] The polypeptides involved in this application (eg, SEQ ID No: 1-6) can be produced by fermentation using Escherichia coli as a host cell. The cDNA sequence encoding the polypeptide was ligated into the pET15b plasmid through enzyme digestion, and then transfected into Escherichia coli. The obtained positive clones were subjected to primary fermentation, and then the primary fermentation liquid was moved to a secondary fermenter (500 L), and IPTG induction was performed when the OD value was 2-5. After protein expression, the mycelia were collected and subjected to cell disruption. After the broken mycelia was collected and resuspended, it was purified with a C 8 liquid phase column and lyophilized for future use.
Embodiment 2
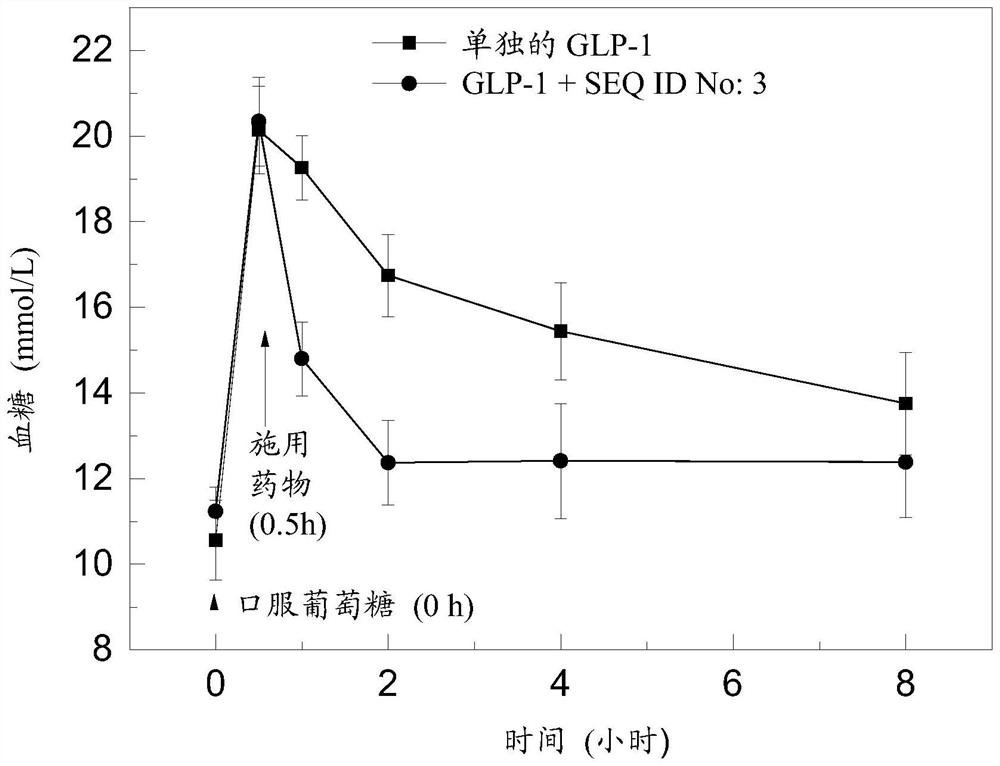
[0216] Embodiment 2: Preparation comprises the pharmaceutical composition of small molecule drug and SEQ ID No:3
[0217] Take amoxicillin, dissolve it in physiological saline to form a 1mmol / L solution and mix well, weigh 0.2mmol of the polypeptide shown in SEQ ID No:3, dissolve it in phosphate buffer (pH=3.0) neutralize and fully mix, and mix amoxicillin with the polypeptide shown in SEQ ID No:3 according to the molar ratio of 5:1. At 4°C, sonicate for 5 minutes, then adjust the pH to 7.0-7.3 to obtain a pharmaceutical composition solution, which is lyophilized for use.
Embodiment 3
[0218] Embodiment 3: Preparation comprises the pharmaceutical composition of siRNA and SEQ ID No:3
[0219] Weigh 3nmol of EGFR siRNA freeze-dried powder and dissolve it in 1mL of phosphate buffer (pH=3.0) and mix well, weigh 9μM of the polypeptide freeze-dried powder shown in SEQ ID No:3 and dissolve it in the above-mentioned containing EGFR siRNA lyophilized powder in phosphate buffer solution and thoroughly mixed, then stirred at 4° C. for 1 hour, and lyophilized to obtain a solid powder of the pharmaceutical composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com