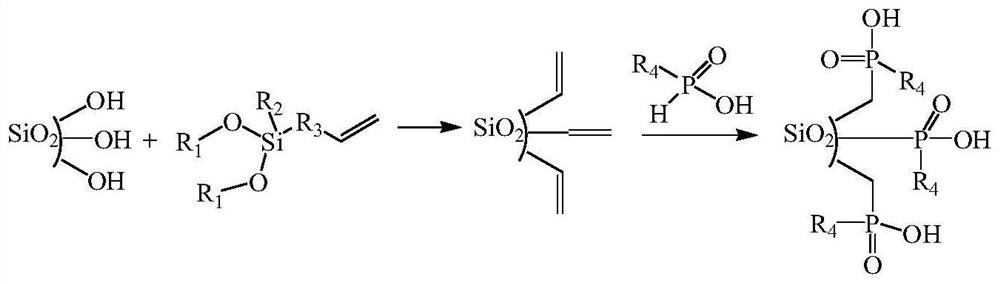
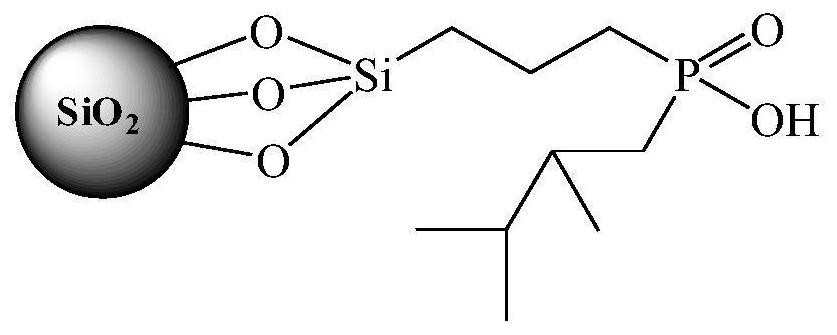
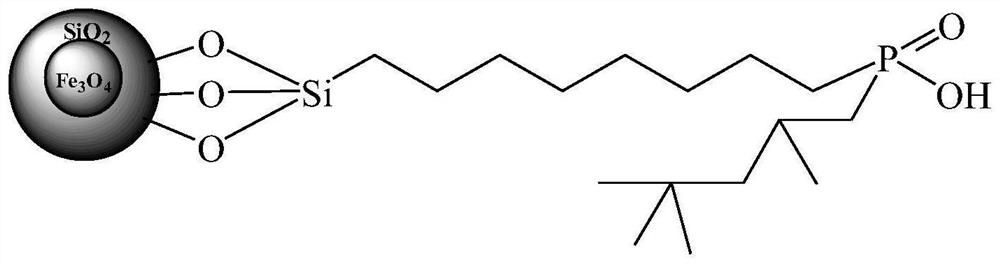
Preparation method of organic phosphinic acid functional group modified silicon-based adsorption material
A technology of phosphinic acid functional and adsorption materials, which is applied in chemical instruments and methods, inorganic chemistry, and other chemical processes, and can solve problems such as inability to absorb, affect the stability and cycle performance of extraction resins, and the loss of extractants. , to achieve the effects of fast adsorption kinetics, simple and easy preparation method, and stable chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Silica gel activation: Reflux 20g of 60-80 mesh silica gel particles in 100mL of 6mol / L HCl for 4h, filter and wash repeatedly with deionized water, dry at 105°C for 10h, and store in a vacuum desiccator for later use.
[0026] (2) Grafting carbon-carbon double bonds on the surface of silica gel: dissolve 6.0 g of activated silica gel and 12 mL of allyltriethoxysilane in 50 mL of deionized water-ethanol mixed solvent (V 水 :V 乙醇 =1:1), reacted at 75°C for 24h. The silica gel particles were centrifuged, washed with ethanol for 3 times, and air-dried at room temperature to obtain silica gel particles grafted with carbon-carbon double bonds on the surface.
[0027] (3) Reaction of silica gel grafted with carbon-carbon double bonds and mono(2,3-dimethylbutyl)phosphinic acid: 6.0 g of silica gel particles grafted with carbon-carbon double bonds on the surface, 1.2 g glacial acetic acid, 3.0 g mono(2,3-dimethylbutyl) phosphinic acid, 0.25 g di-tert-butyl peroxide and 20 ...
Embodiment 2
[0029] (1) Fe 3 o 4 @SiO 2 Grafting carbon-carbon double bonds on the surface of magnetic microspheres: 2.0g Fe 3 o 4 @SiO 2 Magnetic microspheres and 12 mL of trimethoxy(7-octen-1-yl) silane were dissolved in 50 mL of toluene, and reacted at 90° C. for 8 h. Filtration, repeated washing with toluene, and vacuum drying at 50 ° C to obtain Fe with carbon-carbon double bonds grafted on the surface 3 o 4 @SiO 2 magnetic microspheres.
[0030] (2) Fe grafted with carbon-carbon double bonds 3 o 4 @SiO 2 Reaction of magnetic microspheres with mono(2,4,4-trimethylpentyl)phosphinic acid: Measure 2.0g of Fe with carbon-carbon double bonds grafted on the surface in a 250mL three-neck flask 3 o 4 @SiO 2 Magnetic microspheres, 10mL xylene, 0.5mL concentrated sulfuric acid, 6.0g mono(2,4,4-trimethylpentyl)phosphinic acid and 0.25g dibenzoyl peroxide were reacted under reflux for 24h (during Add 0.25 g of dibenzoyl peroxide). After cooling down to room temperature, filter, was...
Embodiment 3
[0032](1) SBA-15 surface grafted carbon-carbon double bond: 1.0g SBA-15 and 1mL methylvinyldiethoxysilane were dissolved in 20mL deionized water-1,4-dioxane mixed solvent ( V 水 :V 1,4-二氧六环 =2:1), reacted at 50°C for 16h. After filtration, repeated washing with 1,4-dioxane, and vacuum drying at 60°C, SBA-15 with carbon-carbon double bonds grafted on the surface was obtained.
[0033] (2) Reaction of SBA-15 grafted with carbon-carbon double bonds and mono(cyclohexyl) phosphinic acid: Measure 1.0 g of SBA-15 grafted with carbon-carbon double bonds on the surface, 0.3 mL of concentrated hydrochloric acid, 1.5g of mono(cyclohexyl)phosphinic acid, 0.10g of azobisisobutyronitrile, 0.10g of di-tert-butyl peroxide and 10mL of 1,4-dioxane, sealed, and reacted at 120°C for 36h (during which every 12h added 0.10g tert-butyl hydroperoxide). After cooling down to room temperature, after filtration and washing with 1,4-dioxane for 3 times, vacuum-dried at 60°C to obtain SBA-15 modified w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com