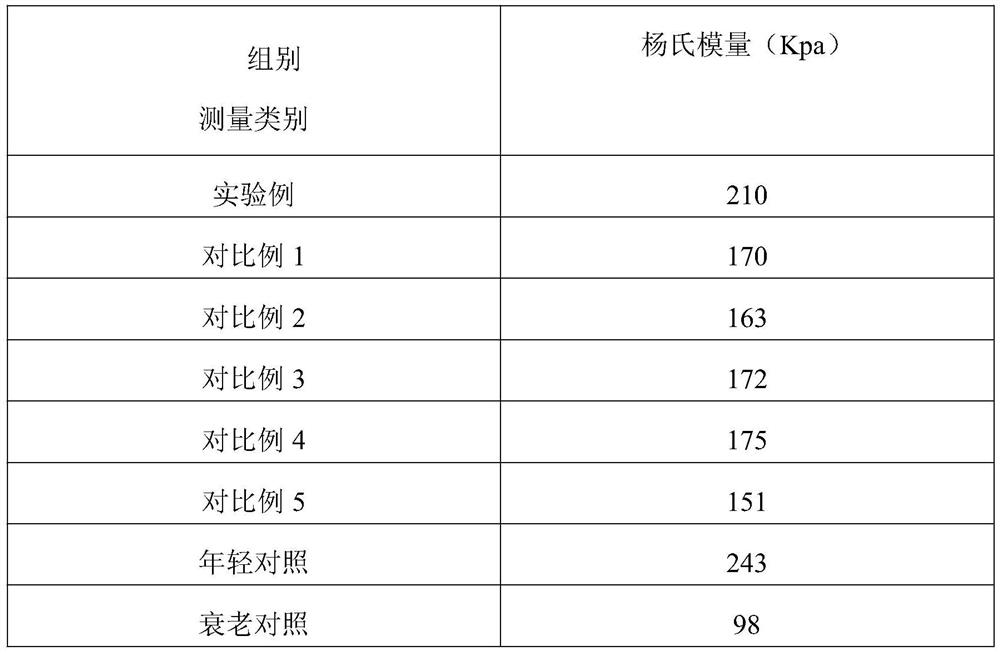
Polypeptide liposome containing fatty acid, and preparation method thereof
A fatty acid and peptide lipid technology, which is applied in the field of fatty acid-containing polypeptide liposomes and its preparation, can solve the problems of liposome drug transport difficulties, destruction of lipid bilayer structure, drug leakage, etc., to improve skin luster, inhibit The effect of shrinking facial muscles and reducing wrinkles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
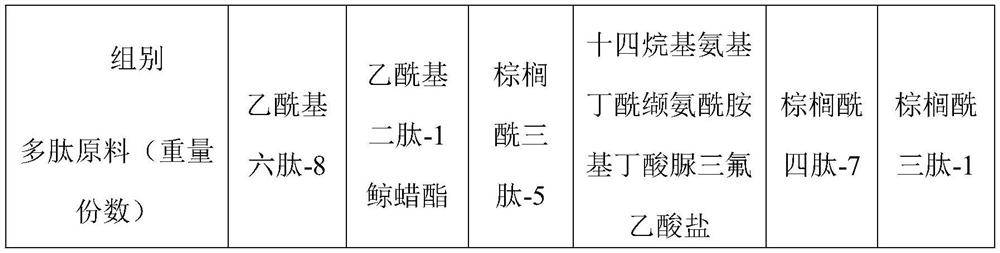
[0028] A fatty acid-containing polypeptide liposome, including an oil phase and a water phase, the oil phase includes the following raw materials in parts by weight: 2-8 parts of hydrogenated lecithin, 0.05-1 part of palmitic acid, 0.2-0.8 parts of stearic acid, 0.05-1 part of behenic acid and 15-25 parts of caprylic / capric triglyceride; the water phase includes the following raw materials in parts by weight: 0.05-1 part of acetyl hexapeptide-8, 0.05-1 part of acetyl dipeptide- 1 cetyl ester, 0.05-1 part palmitoyl tripeptide-5, 0.05-1 part tetradecylaminobutyryl valinyl amidobutyrate urea trifluoroacetate, 10-30 parts glycerin, 5-15 parts Butanediol and 39.5-43.3 parts of water.
[0029] The PC content of the hydrogenated lecithin is 50-75%.
[0030] The preparation method of its polypeptide liposome comprises the following steps:
[0031] S1. Mix hydrogenated lecithin, palmitic acid, stearic acid, behenic acid, caprylic acid / capric triglyceride, heat to 65-85°C, stir and di...
Embodiment 2
[0035] A fatty acid-containing polypeptide liposome, comprising an oil phase and an aqueous phase, the oil phase comprising the following raw materials in parts by weight: 2 parts of hydrogenated lecithin, 0.05 part of palmitic acid, 0.2 part of stearic acid, 0.05 part of behenic acid and 15 parts of caprylic / capric triglyceride; the water phase includes the following raw materials in parts by weight: 0.05 part of acetyl hexapeptide-8, 0.05 part of acetyl dipeptide-1 cetyl ester, 0.05 part of palmitoyl tripeptide-5, 0.05 parts tetradecylaminobutyryl valinyl amidobutyrate urea trifluoroacetate, 10 parts glycerin, 5 parts butylene glycol and 39.5 parts water.
[0036] The PC content of the hydrogenated lecithin is 50%.
[0037] The preparation method of its polypeptide liposome comprises the following steps:
[0038] S1. Mix hydrogenated lecithin, palmitic acid, stearic acid, behenic acid, caprylic / capric triglyceride, heat to 65°C and stir to dissolve for 2 hours to obtain the...
Embodiment 3
[0042] A fatty acid-containing polypeptide liposome, comprising an oil phase and an aqueous phase, the oil phase comprising the following raw materials in parts by weight: 8 parts of hydrogenated lecithin, 1 part of palmitic acid, 0.8 part of stearic acid, 1 part of behenic acid and 25 parts of caprylic / capric triglyceride; the water phase includes the following raw materials in parts by weight: 1 part of acetyl hexapeptide-8, 1 part of acetyl dipeptide-1 cetyl ester, 1 part of palmitoyl tripeptide-5, 1 part tetradecylaminobutyryl valinyl amidobutyrate urea trifluoroacetate, 30 parts glycerin, 15 parts butylene glycol, and 43.3 parts water.
[0043] The PC content of the hydrogenated lecithin is 60%.
[0044] The preparation method of its polypeptide liposome comprises the following steps:
[0045] S1. Mix hydrogenated lecithin, palmitic acid, stearic acid, behenic acid, and caprylic / capric triglyceride, heat to 85° C., stir and dissolve for 0.5 h, and obtain the oil phase; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com