Preparation method of sitagliptin intermediate
A sitagliptin and intermediate technology, applied in the field of enzyme engineering and biopharmaceuticals, can solve the problems of high difficulty in product separation and purification, low product yield, high production cost, etc., simplify the product separation and purification process, and increase product yield and organic solvent recovery, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
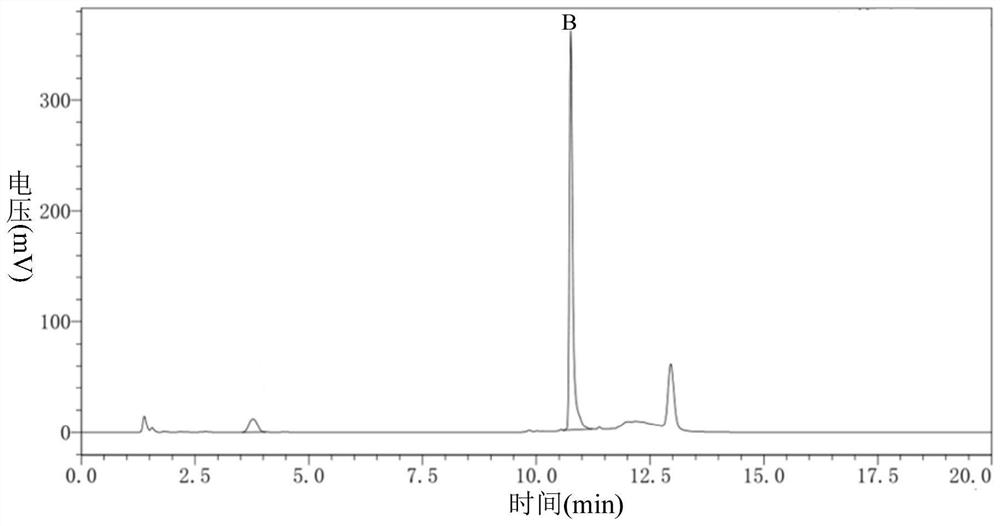
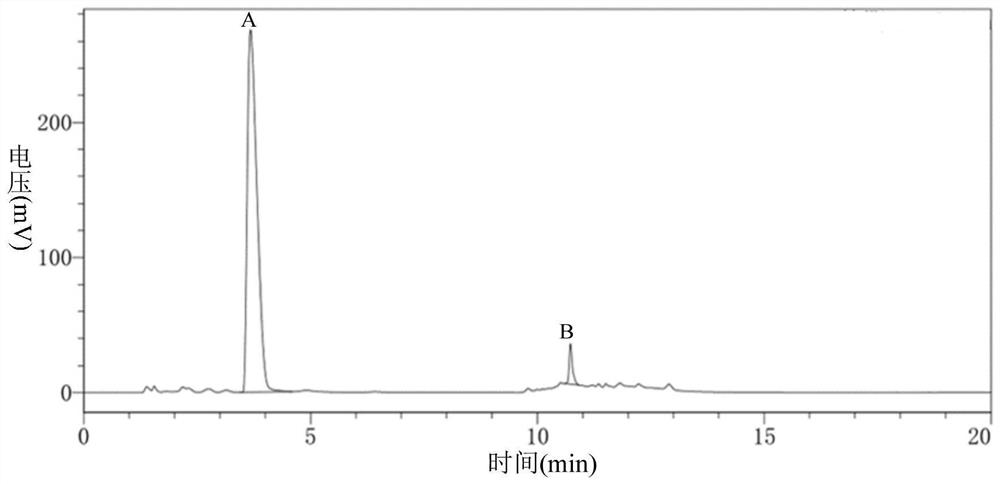
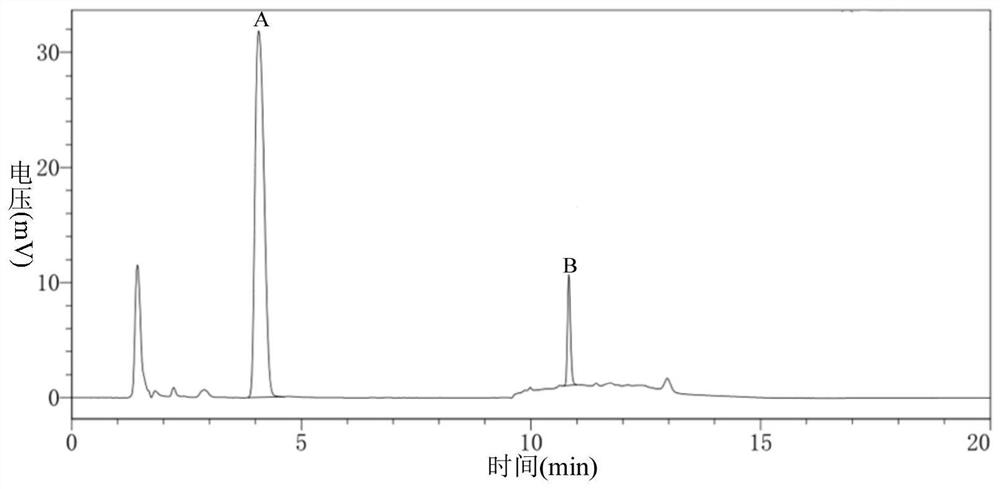
Image
Examples
preparation example Construction
[0042] The embodiment of the present application provides a preparation method of a sitagliptin intermediate, and a sitagliptin intermediate prepared by the preparation method, the preparation method comprising the following steps:
[0043] S a , Sitagliptin precursor ketone (2Z)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro-[1,2,4] shown in formula (I) ]Triazolo[4,3-a]pyrazin-7-(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one as substrate, with in a standard Organic solvents having a boiling point of not higher than 110°C under atmospheric pressure are mixed to obtain a mixed solution; and
[0044] S b 1. Conduct transamination contact between the mixed solution and transaminase to generate an enzyme-catalyzed reaction to generate the sitagliptin intermediate represented by formula (II).
[0045] In some embodiments, step S b The method of transaminating the mixed solution with the transaminase is as follows: all the mixed solution is directly mixed with the transaminase. As an ...
Embodiment 1
[0079] Embodiment 1: provide the transaminase used for transaminase catalyzed reaction
[0080] The embodiment of the present application provides a total of five transaminases (corresponding to numbers 1 to 5), wherein the transaminases numbered 1 to 4 are obtained by one or more point mutations of the transaminase having the amino acid sequence shown in SEQ ID NO: 1 , wherein, the nucleotide sequence of the gene encoding the transaminase having the amino acid sequence shown in SEQ ID NO: 1 is shown in SEQ ID NO: 2, and the specific information of the transaminases numbered 1 to 5 is shown in Table 1 below:
[0081] Table 1 Mutation mode and sequence information of transaminases No. 1 to No. 5
[0082]
[0083] 1.1. Construction of genetically engineered bacteria containing transaminase coding genes
[0084] Construct the genetically engineered bacteria containing the transaminase coding gene of No. 1 to No. 5 respectively, wherein, the steps of constructing the genetical...
Embodiment 2
[0104] Embodiment 2: select the used organic solvent of transaminase catalyzed reaction
[0105] The transaminase enzyme solutions No. 1 to No. 5 prepared in Example 1 were subjected to an organic solvent screening experiment.
[0106] 2.1. Organic solvent single agent experiment
[0107] The method flow of organic solvent single agent experiment is as follows:
[0108] S2.1.1. Take 100 μL of the single transaminase solution prepared in Example 1 (transaminase solution No. 1, transaminase solution No. 2, transaminase solution No. 3, transaminase solution No. 4, or transaminase solution No. 5) Transaminase enzyme liquid) is placed on the reaction plate, then in the transaminase enzyme liquid, add 1.5mL pH to be the stock mixture of 8.5, wherein, the stock mixture comprises the triethanolamine of 0.2mol / L, the pyridoxal phosphate (PLP) of 2mmol / L , 2mol / L isopropylamine (IPM) and sterile water to obtain a premixed system;
[0109] S2.1.2. Add 400 μL of the mixed solution to t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com