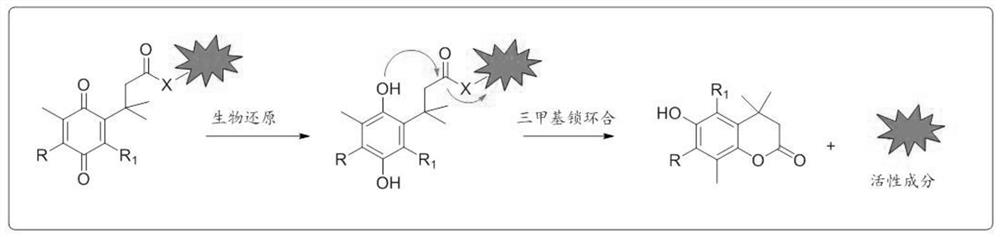
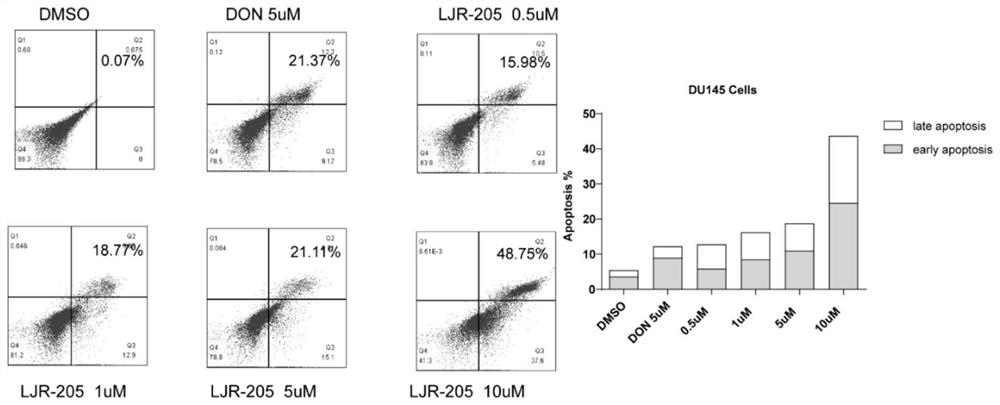
NQO1 activated 6-diazo-5-oxo-L-n-leucine prodrug as well as preparation method and application thereof
A norleucine, diazo group technology, applied in the field of medicinal chemistry, can solve the problems of uneven biological distribution, limited application, limited clinical use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Compound (S)-6-diazo-2-(3-methyl-3-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-diene-1- Synthesis of ethyl)butanylamino)-5-oxohexanoate (LJR-201)
[0084] Dissolve compound 3e (1g, 3.99mmol) in DCM, add HATU (1.78g, 4.69mmol), DIPEA (1.83g, 14.08mmol), stir at room temperature (26°C) for 0.5 hours, then slowly add compound 5a (0.80g , 4.0 mmol), after stirring at room temperature for 4 hours, the solvent was evaporated under reduced pressure, separated and purified by column chromatography (PE:EA=3:1) to obtain a light yellow solid LRJ-201 (1.07g). Yield 62%. mp=107-109°C. 1 H NMR (300MHz, CDCl 3 ):δ=6.28(d,1H,J=6.0Hz),5.30-5.26(m,1H),4.47-4.40(m,1H),4.18-4.11(m,2H),2.83(s,2H), 2.36-2.33(m,2H),2.11(s,3H),1.95(d,6H,J=6.0Hz),1.42(s,6H),1.26(t,3H,J=6.0Hz)ppm. 13 C NMR (75MHz, CDCl 3 ):δ=193.74,191.11,187.56,171.90,171.68,153.05,143.42,137.99,137.88,125.23,69.2,66.2,61.62,51.59,48.96,38.28,36.49,28.92,28.80,14.11,12.70,12.13ppm.HRMS (ESI + ):m / z[M+H] +calcd for C 22 h...
Embodiment 2
[0086] Compound (S)-6-diazo-2-(3-(4,5-dimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)-3-methyl Synthesis of ethyl butyramide-5-oxohexanoate (LJR-202)
[0087] Dissolve compound 3f (0.94g, 3.99mmol) in DCM, add HATU (1.78g, 4.69mmol), DIPEA (1.83g, 14.08mmol), stir at room temperature (26°C) for 0.5 hours, then slowly add compound 5a (0.80 g, 4.0mmol), stirred at room temperature (26°C) for 4 hours, evaporated the solvent under reduced pressure, separated and purified by column chromatography (PE:EA=1:1) to obtain a yellow solid LJR-202 (0.85g), yield 51 %. 1 H NMR (300MHz, CDCl 3 )δ=6.48(s,1H),6.23(d,1H,J=9.0Hz),5.24(s,1H),4.44-4.37(m,1H),4.17-4.10(m,2H),2.86-2.73 (m,2H),2.36-2.31(m,2H),2.13(s,1H),2.04-1.98(m,7H),1.61(s,6H),1.32-1.24(m,3H)ppm.HRMS( ESI + ):m / z[M+H] + calcd for C 21 h 28 N 3 o 6 + , 418.1973; found. 418.1965.
Embodiment 3
[0089] Compound (S)-2-(3-(5-bromo-2,4-dimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)-3-methylbutane Synthesis of ethyl amido)-6-diazo-5-oxohexanoate (LJR-203)
[0090] Dissolve compound 3g (1.25g, 3.99mmol) in DCM, add EDCI (0.89g, 4.69mmol), HOBT (0.62g, 4.69mmol), stir at room temperature (26°C) for 0.5 hours, then slowly add compound 5a (0.80 g, 4.0mmol), stirred at room temperature (26°C) for 3 hours, evaporated the solvent under reduced pressure, separated and purified by column chromatography (PE:EA=3:1) to obtain a brown solid LJR-203 (0.66g), and the yield was 33%. mp=115-117°C. 1 H NMR (300MHz, CDCl 3 ):δ=6.27(d,1H,J=9.0Hz),5.31(d,1H,J=6.0Hz),4.49-4.42(m,1H),4.19-4.12(m,2H),3.14-3.09( m,1H),2.80(s,1H),2.70-2.65(m,1H),2.37-2.34(m,2H),2.16(s,6H),2.04-1.94(m,2H),1.44(d, 6H,J=9.0Hz),1.27-1.25(m,3H)ppm. 13 C NMR (75MHz, CDCl 3 ):δ=193.95,184.70,182.46,171.72,171.68,153.86,143.74,138.09,137.03,136.52,61.66,55.18,51.66,49.01,38.82,36.6,28.90,17.10,146.4MS + ):m / z[M+H] +...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com