Preparation method of chlorpromazine hydrochloride
A technology of chlorpromazine hydrochloride and chlorpromazine, which is applied in the direction of organic chemistry, can solve the problems of complex reaction process, low production efficiency, and large quality difference, so as to improve yield and purity, improve production efficiency, and facilitate recycling Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
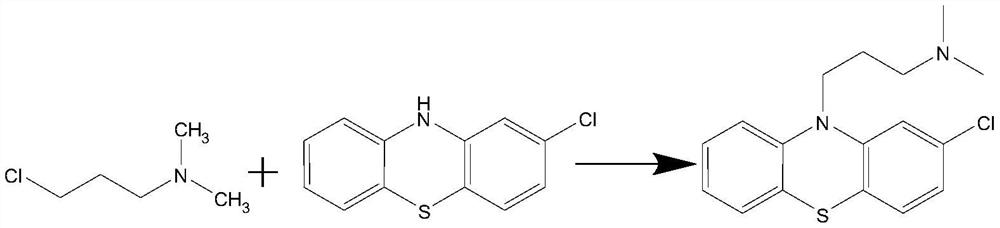
[0026] (1) According to the mass ratio of 1:1.5, put 2 8 Chlorophenothiazine and N-methylpyrrolidone, stirring and heating to reflux, remove 2 8 Water in the chlorophenothiazines, kept at reflux for 2 hours;
[0027] (2) Add 4-dimethylaminopyridine under reflux, add dropwise the N-methylpyrrolidone solution containing N,N-dimethyl-3-chloropropylamine, continue to reflux after dripping; keep reflux for 2 hours; 2- The mass ratio of chlorophenothiazine to N,N-dimethyl-3-chloropropylamine and 4-dimethylaminopyridine is 1:1.0:0.4.
[0028] (3) After the reaction is finished, cool to below 50°C, slowly add 30% water to the reaction solution volume, let it stand for 1 hour, separate the water layer, wash the oil layer with hot water at 55°C, stir for 15 minutes, and let stand for more than 30 minutes, Then divide the water layer;
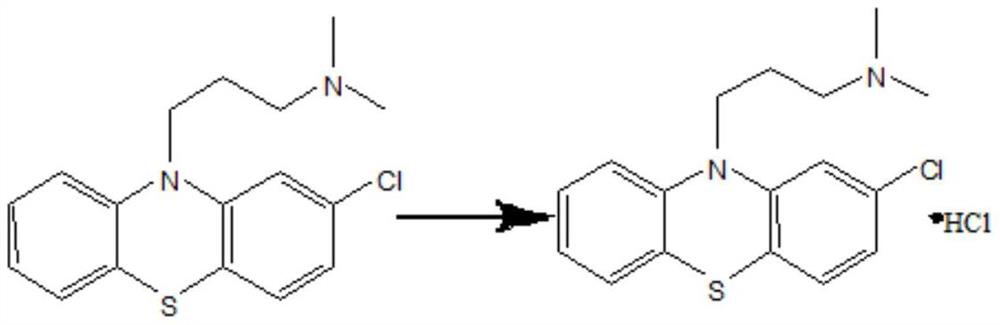
[0029] (4) Stir the oil layer and add hydrochloric acid to acidify, acidify to PH value 2 as the end point, and let stand to separate; add N-methylpyr...
Embodiment 2
[0036] (1) According to the mass ratio of 1:2.0, put 28 chlorophenothiazine and N-methylpyrrolidone into a dry and clean reaction pot, stir and heat to reflux, remove the water in 28 chlorophenothiazine; keep reflux for 3 hours;
[0037] (2) Add 4-dimethylaminopyridine under reflux, add dropwise the N-methylpyrrolidone solution containing N,N-dimethyl-3-chloropropylamine, continue to reflux after dripping; keep reflux for 2 hours; 2- The mass ratio of chlorophenothiazine to N,N-dimethyl-3-chloropropylamine and 4-dimethylaminopyridine is 1:0.8:0.5.
[0038] (3) Cool down to below 50°C after the reaction, slowly add 50% water to the reaction solution volume, let stand for 1 hour, separate the water layer, wash the oil layer with 55°C hot water, stir for 15 minutes, let stand for more than 30 minutes, Then divide the water layer;
[0039] (4) Stir the oil layer and add hydrochloric acid to acidify, acidify to PH value 2 as the end point, and let stand to separate; add N-methylpy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com