Method for determining gallium in gallium arsenide wafer production wastewater
A technology for gallium arsenide wafers and waste water production, which is applied in the field of analytical chemistry and can solve problems such as blanks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
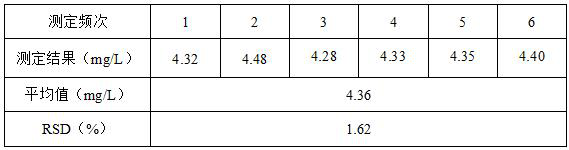
[0035] Embodiment 1: Determination of gallium in 1# gallium arsenide wafer production wastewater
[0036] Pipette 50mL (denoted as V) of gallium arsenide wafer production wastewater from a certain enterprise into a 250mL beaker, add 5mL of 1+1 sulfuric acid, add 5mL of concentrated hydrochloric acid after a little heating, evaporate to 20mL at a low temperature of 40~60℃, and then add concentrated Hydrochloric acid 5mL, repeated evaporation 3 times to remove volatile elements such as arsenic and tin in the wastewater. Until it is nearly dry after evaporating and emitting thick smoke of sulfur trioxide, then add 5.0mL of dilute hydrochloric acid with a concentration of 6mol / L to dissolve the salts, and finally, set the volume to 100mL (denoted as V 1 ) volumetric flask shake well to get the test solution.
[0037] Draw 10.00mL (denoted as V 2 ) test solution in a 25mL colorimetric tube, dropwise add titanium trichloride solution with a mass concentration of 15% to purple, the...
Embodiment 2
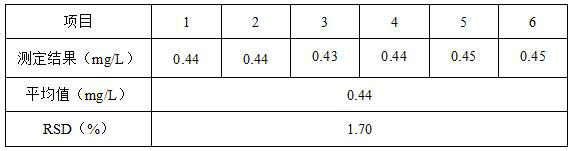
[0042] Example 2: Determination of gallium in 2# gallium arsenide wafer production wastewater and verification of the precision of the method
[0043] Pipette 50mL of gallium arsenide wafer production wastewater from a certain company into a 250mL beaker, add 10mL of 1+1 sulfuric acid, add 5mL of concentrated hydrochloric acid after a little heating, evaporate to 30mL at a low temperature of 40~60℃, then add 5mL of concentrated hydrochloric acid, and evaporate repeatedly 4 times to remove volatile elements such as arsenic and tin in wastewater. After evaporating and emitting thick smoke of sulfur trioxide, it is nearly dry, then add 7.0mL of dilute hydrochloric acid with a concentration of 6mol / L to dissolve the salts, and finally, dilute to a 200mL volumetric flask and shake well to obtain a test solution.
[0044] Draw 5.00mL of test solution into a 25mL colorimetric tube, dilute to 10mL with 6mol / L dilute hydrochloric acid, add dropwise 20% titanium trichloride solution to ...
Embodiment 3
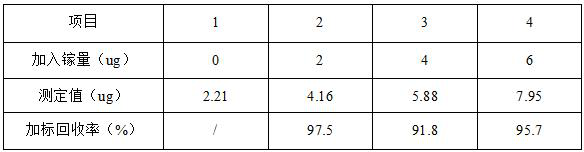
[0049] Example 3: Gallium Determination and Method Addition Recovery Experiment of 3# Gallium Arsenide Wafer Production Wastewater
[0050] Pipette three parts of 50mL of gallium arsenide wafer production wastewater in Example 1 into 250mL beakers, add gallium standard solution (1mg / L) 2.00, 4.00, 6.00mL respectively, add 1+1 sulfuric acid 5mL, slightly heat, add Concentrated hydrochloric acid 5mL, low-temperature evaporation to 20mL, then add 5mL concentrated hydrochloric acid, repeated evaporation three times, in order to remove arsenic, tin and other volatile elements in the wastewater. Until it is nearly dry after evaporating and emitting thick smoke of sulfur trioxide, then add 5.0mL of dilute hydrochloric acid with a concentration of 6mol / L to dissolve the salts, and finally, dilute to a 100mL volumetric flask and shake well to obtain a test solution.
[0051] Draw 10.00mL of the test solution into a 25mL colorimetric tube, add dropwise a 15% titanium trichloride solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com