Photo-oxidation reduction catalysis method
A technology of photooxidation and compounds, applied in organic chemical methods, chemical instruments and methods, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] A preparation method of 2-(1,2-diphenylethyl) malononitrile compound (structural formula shown in formula 1 below):
[0085]
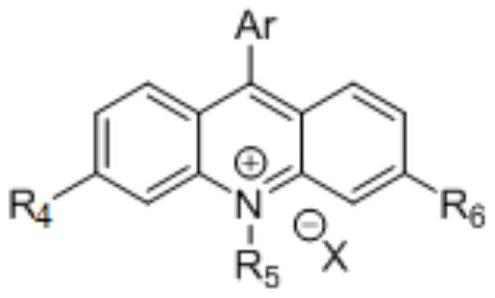
[0086] Photocatalyst Mes-Acr-PhBF 4 (0.01 mmol, 4.6 mg) and capture reagent benzylmalononitrile (0.4 mmol, 61.7 mg) were weighed into oven-dried 8 mL vials equipped with magnetic star marking rods. Anhydrous acetonitrile (1 mL) was added followed by the linear tertiary alcohol (2-methyl-1-phenyl-2-propanol) (0.2 mmol). The reaction vessel was degassed, backfilled with argon, and then placed in a SynLED 4x4 photoreactor (SynLED discoverTM 450nm, designed and manufactured by Shenzhen SynLED Tech. Ltd). The progress of the reaction was monitored by TLC. After completion, the reaction mixture was concentrated and purified by silica gel flash column chromatography to obtain the target product in 85% yield.
[0087] Correlation characterization analysis, the result is 1 H NMR (500MHz, Chloroform-d) δ7.47–7.38(m,5H),7.35(dd,J=8.1,6.4Hz,2H),7.32–...
Embodiment 2
[0089] A preparation method of 2-(1,3-diphenylpropyl) malononitrile compound (structural formula shown in formula 2 below):
[0090]
[0091] The linear tertiary alcohol is 3-methyl-1-phenyl-3-pentanol, and the capture reagent is benzallyl dinitrile; other preparation methods are the same as in Example 1, and the yield is 81%.
[0092] Correlation characterization analysis, the result is 1 H NMR (500MHz, Chloroform-d) δ7.51–7.40(m,3H),7.38–7.28(m,4H),7.26–7.22(m,1H),7.14–7.07(m,2H),3.86(d ,J=6.2Hz,1H),3.20(dt,J=10.2,5.7Hz,1H),2.66(ddd,J=13.6,8.3,5.2Hz,1H),2.48(dt,J=13.8,8.2Hz, 1H),2.43–2.29(m,2H). 13 C NMR (126MHz, Chloroform-d) δ139.90, 136.24, 129.48, 129.12, 128.73, 128.38, 128.05, 126.57, 111.81, 111.77, 45.64, 33.53, 32.77, 30.37. HRMS (ESI-TOF) calculated for C 18 h 16 N 2 (M+Na + ): 283.1206, found: 283.1206. This result further confirms that the molecular structure of the product is just like the molecular structure 2 above.
Embodiment 3
[0094] A preparation method of 2-(2-(4-isobutylphenyl)-1-phenylpropyl) malononitrile compound (structural formula shown in formula 3 below):
[0095]
[0096] Linear tertiary alcohol is 3-(4-isobutylphenyl)-2-methylbutan-2-alcohol, and trapping reagent is benzallyl dinitrile; Others are identical with the preparation method of embodiment 1, and productive rate is 84%, dr is 1:1.
[0097] Correlation characterization analysis, the result is isomer 1: 1 H NMR (500MHz, Chloroform-d) δ7.31–7.22(m,3H),7.01–6.90(m,4H),6.84(d,J=7.9Hz,2H),4.07(d,J=8.1Hz, 1H), 3.49(p, J=7.0Hz, 1H), 3.42(t, J=7.8Hz, 1H), 2.40(dd, J=7.2, 1.8Hz, 2H), 1.80(dp, J=13.5, 6.8 Hz,1H),1.43(d,J=6.9Hz,3H),0.86(dd,J=6.6,1.5Hz,6H). 13 C NMR (126MHz, Chloroform-d) δ140.84, 137.54, 134.88, 129.12, 128.85, 128.57, 128.46, 127.87, 112.58, 112.03, 52.76, 44.95, 41.25, 30.15, 27.48, 22.38, 192.92
[0098] Isomer 2: 1 H NMR (400MHz, Chloroform-d) δ7.58–7.42 (m, 5H), 7.30 (d, J = 7.9Hz, 2H), 7.23 (d, J = 8.1Hz, 2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com