Solvent circulation preparation method and application of a metal atom-level dispersed nitrogen-carbon material
A technology of solvent circulation and metal nitrogen, applied in the direction of structural parts, electrolytic organic production, electrolytic components, etc., can solve the problems of not meeting the requirements of green chemistry, restricting industrial production, and low utilization of atoms, and achieve simple and easy reaction operation, The effect of low price and avoiding complicated processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A solvent cycle preparation method of metal atomic dispersed nitrogen-carbon material, the preparation method comprises the following steps:
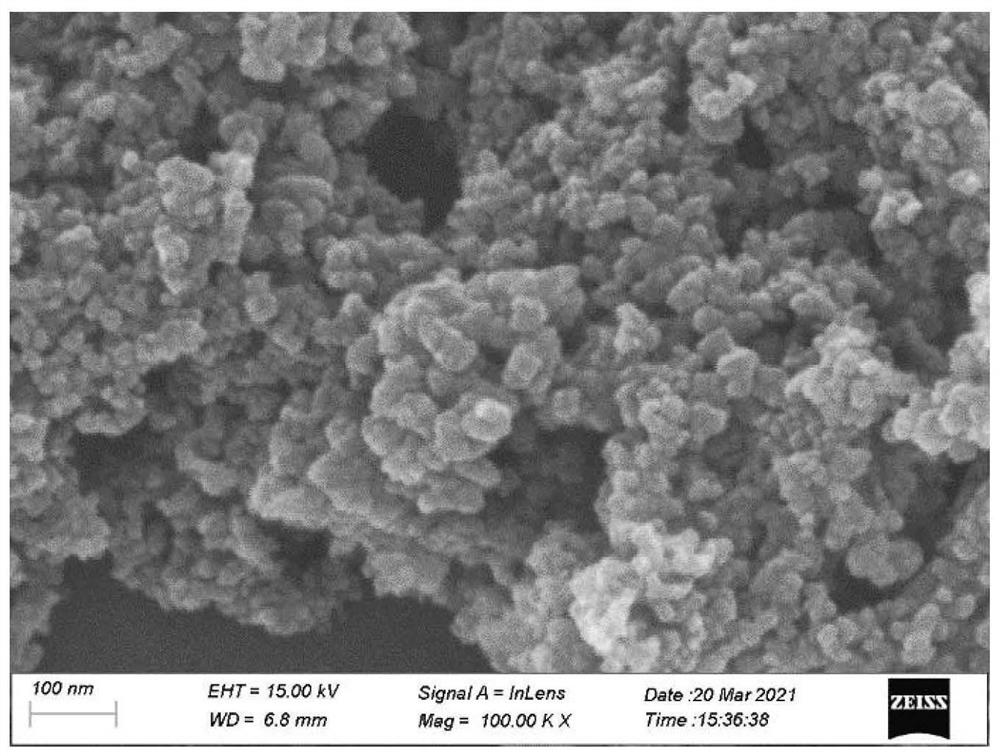
[0042] Weigh 3 mmol of zinc powder and dissolve it in 30 ml of formamide. After stirring at 60 degrees Celsius for 0.5 hours, ultrasonically disperse for 0.5 hours. Alternately perform high-temperature stirring and ultrasonic dispersion until a golden yellow clear and transparent solution is obtained. Transfer this solution to In a 40 ml hydrothermal reactor, the reaction was carried out at 220 degrees Celsius for 12 hours. Then, the product solution was suction filtered to separate the solid and the formamide solution L1, the solid was washed three times with deionized water, and the obtained filter cake was freeze-dried in a vacuum freeze dryer to obtain powder materials. After the powder material is ground and calcined at 600 degrees Celsius in an argon atmosphere for 4 hours, the black powder material obtained is the metal ni...
Embodiment 2
[0050] The difference from Example 1 is:
[0051] 3 mmol of zinc powder was changed to 10 mmol of zinc acetate, and the reaction process of solvent circulation operation was: in a 40 ml hydrothermal reactor, solvothermal reaction at 160 degrees Celsius for 20 hours to obtain metal nitrogen carbon material ZnNC-2#, Others are the same as in Example 1, and the solvent circulation operation is carried out to the first, second, and third times of Example 1, which are respectively named as metal nitrogen carbon material ZnNC-2#cycling 1st, metal nitrogen carbon material ZnNC-2# Cycling 2nd, metal nitrogen carbon material ZnNC-2#cycling 3rd.
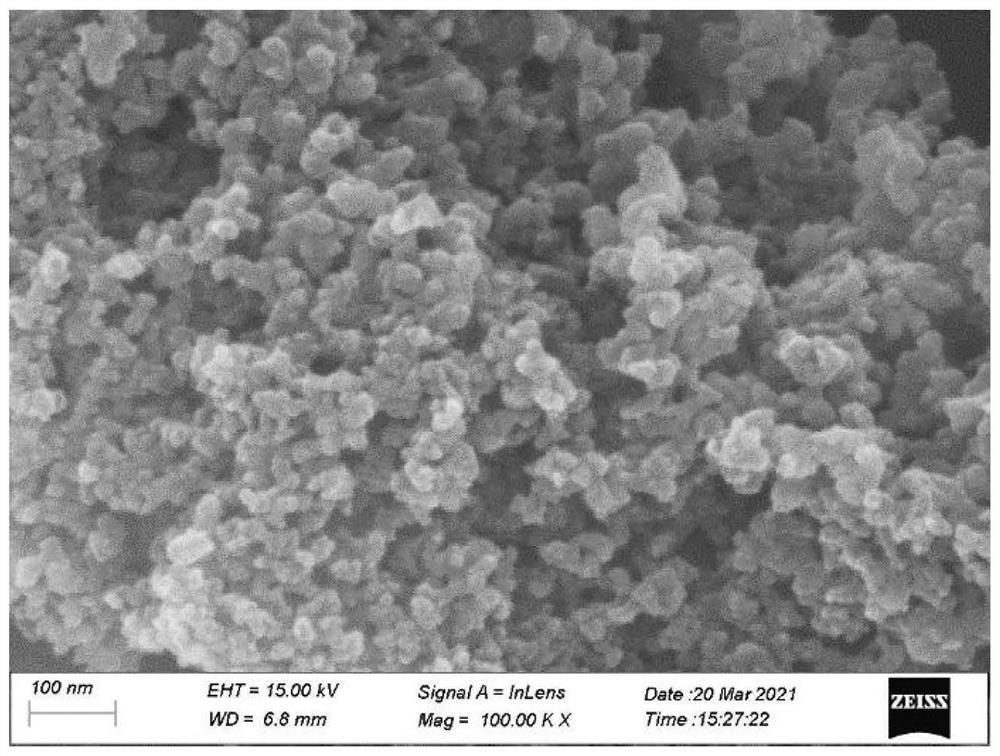
[0052] figure 2 This is the SEM image of the metal nitrogen carbon material ZnNC-2#cycling 2nd prepared in Example 2 of the present invention. , the metal nitrogen carbon material is spherical nanoparticles with a size distribution of about 10 nm.
[0053] Figure 7 This is the XRD image of the metal nitrogen carbon material ZnNC-2#cyclin...
Embodiment 3
[0056] Except that 3 mmol of zinc powder was changed to 0.03 mmol of cobalt acetate and 3 mmol of zinc acetate, and the carbonization temperature was 700 degrees Celsius, the others were the same as in Example 1, and the metal nitrogen carbon material ZnCoNC was prepared, and the solvent circulation operation was carried out to implement In the first, second and third times of Example 1, the metal nitrogen carbon material ZnCoNC cycling 1st, the metal nitrogen carbon material ZnCoNC cycling 2nd, and the metal nitrogen carbon material ZnCoNC cycling 3rd were obtained, respectively.
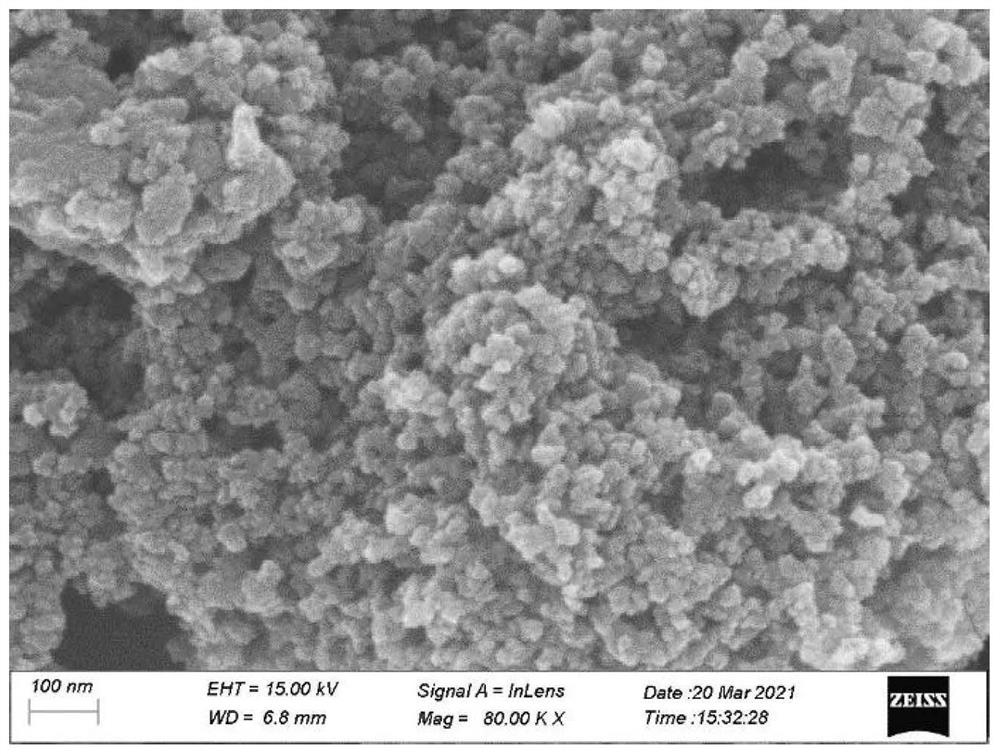
[0057] image 3 This is the SEM image of the metal nitrogen carbon material ZnCoNC prepared in Example 3 of the present invention. The metal nitrogen carbon material is spherical nanoparticles with a size distribution of about 10 nm.
[0058] Figure 8 This is the XRD image of the metal nitrogen carbon material ZnCoNC prepared in Example 3 of the present invention. It can be seen from the figure ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com