Preparation method of trans-3,5-dimethylpiperidine
A technology of dimethylpiperidine and lutidine, which is applied in the field of preparation of trans-3,5-dimethylpiperidine, can solve the problem of low proportion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation method of the trans 3,5-dimethylpiperidine of the present embodiment comprises the following steps:
[0030] A. Under hydrogen protection conditions, add 100g3,5-lutidine, 40g deionized water, 5g composite catalyst (the mass ratio of ruthenium carbon, nickel powder and metal iron acetate is 1:0.05:0.05) to the high pressure reaction React in the still, the reaction pressure of carrying out reaction is 30kg / cm 2 , the reaction temperature is 140°C, the reaction time is 5h, and after the reaction is completed, the crude product of 3,5-dimethylpiperidine is obtained by suction filtration;
[0031] B. Cool the crude 3,5-dimethylpiperidine obtained in step A to room temperature, take out the reaction mixture, and take the supernatant after standing still to obtain 3,5-dimethylpiperidine.
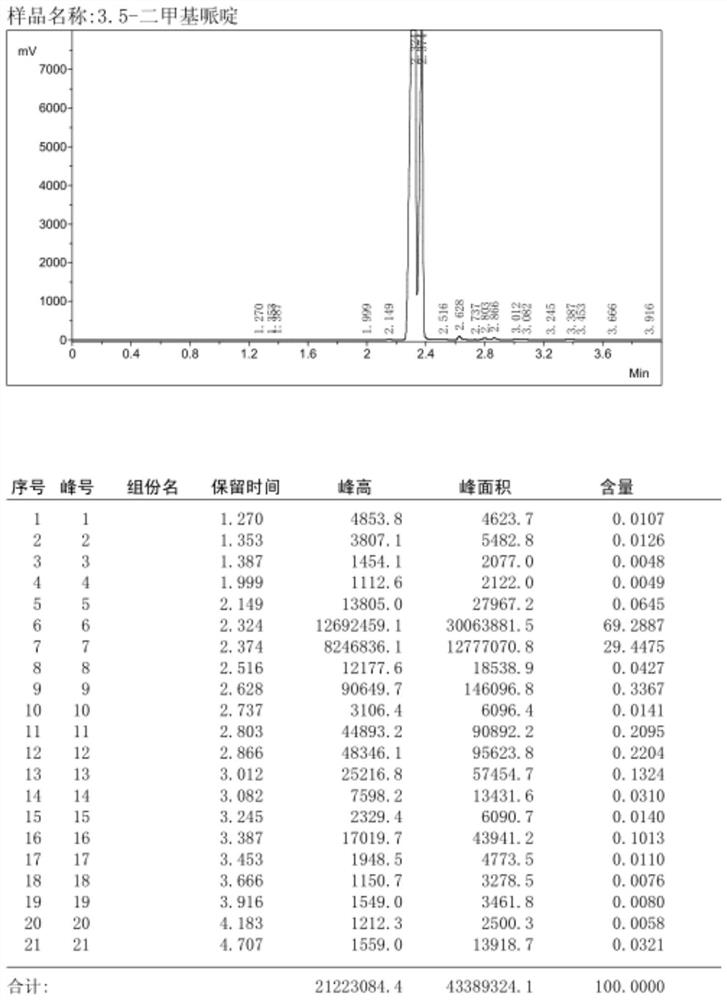
[0032] Since the product 3,5-dimethylpiperidine and the raw material 3,5-lutidine have a low boiling point, a relatively simple and fast gas chromatography method is adopt...
Embodiment 2
[0053] The preparation method of the trans 3,5-dimethylpiperidine of the present embodiment comprises the following steps:
[0054] A. Under hydrogen protection conditions, add 100g3,5-lutidine, 60g deionized water, 8g composite catalyst (the mass ratio of ruthenium carbon, nickel powder and metal zinc acetate is 1:0.08:0.08) to the high pressure reaction React in the still, the reaction pressure of carrying out reaction is 40kg / cm 2 , the reaction temperature is 150°C, the reaction time is 8h, and after the reaction is completed, the crude product of 3,5-dimethylpiperidine is obtained by suction filtration;
[0055] B. Cool the crude 3,5-dimethylpiperidine obtained in step A to room temperature, take out the reaction mixture, and take the supernatant after standing still to obtain 3,5-dimethylpiperidine.
[0056] The content of the trans isomer of the 3,5-dimethylpiperidine mixture detected by gas chromatography (GC) was 34.9%.
Embodiment 3
[0058] The preparation method of the trans 3,5-dimethylpiperidine of the present embodiment comprises the following steps:
[0059] A. Under hydrogen protection conditions, add 100g3,5-lutidine, 50g deionized water, 6g composite catalyst (the mass ratio of ruthenium carbon, nickel powder and metal magnesium acetate is 1:0.1:0.05) to the high pressure reaction React in the still, the reaction pressure of carrying out reaction is 35kg / cm 2 , the reaction temperature is 160°C, the reaction time is 6h, and after the reaction is completed, the crude product of 3,5-dimethylpiperidine is obtained by suction filtration;
[0060] B. Cool the crude 3,5-dimethylpiperidine obtained in step A to room temperature, take out the reaction mixture, and take the supernatant after standing still to obtain 3,5-dimethylpiperidine.
[0061] The content of the trans isomer of the 3,5-dimethylpiperidine mixture detected by gas chromatography (GC) was 32.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com