Medicine composition for preventing and treating swine envelope virus infectious diseases, preparation method of medicine composition, and application of plant extract
A plant extract, infectious disease technology, application in medicine, pharmaceutical composition and preparation field for preventing porcine enveloped virus infectious disease, can solve problems such as unsatisfactory immune effect of vaccines and influence on protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] Among the present invention, the preparation method of zinc-rich inactivated lactic acid bacteria bacterial agent is as follows: 1) Lactobacillus acidophilus carries out strain preparation earlier, and strain screening uses solid MRS medium as basic medium, adds 0.1% CaCO 3 , the pH value is 6.4, the medium is sterilized at 121°C for 20-30 minutes, cooled to 37°C, and set aside; 2) The fermentation culture of Lactobacillus acidophilus, the fermentation medium uses liquid MRS medium as the basic medium, The pH value is 6.4, the medium is sterilized at 121°C for 20-30 minutes, cooled to 37°C, and 0.12% ZnSO is added 4 , mix well, and set aside, the seed age is 12-18 hours, the inoculation amount is 3%-5% of V / V or V / W, the fermentation time is 36-48 hours, the fermentation temperature is 37°C, and the pH value is maintained at about 5.5; 3) Add 0.1-0.5% (w / v) of a protective agent to the fermentation broth after the fermentation is finished, and the protective agent is sk...
Embodiment 1
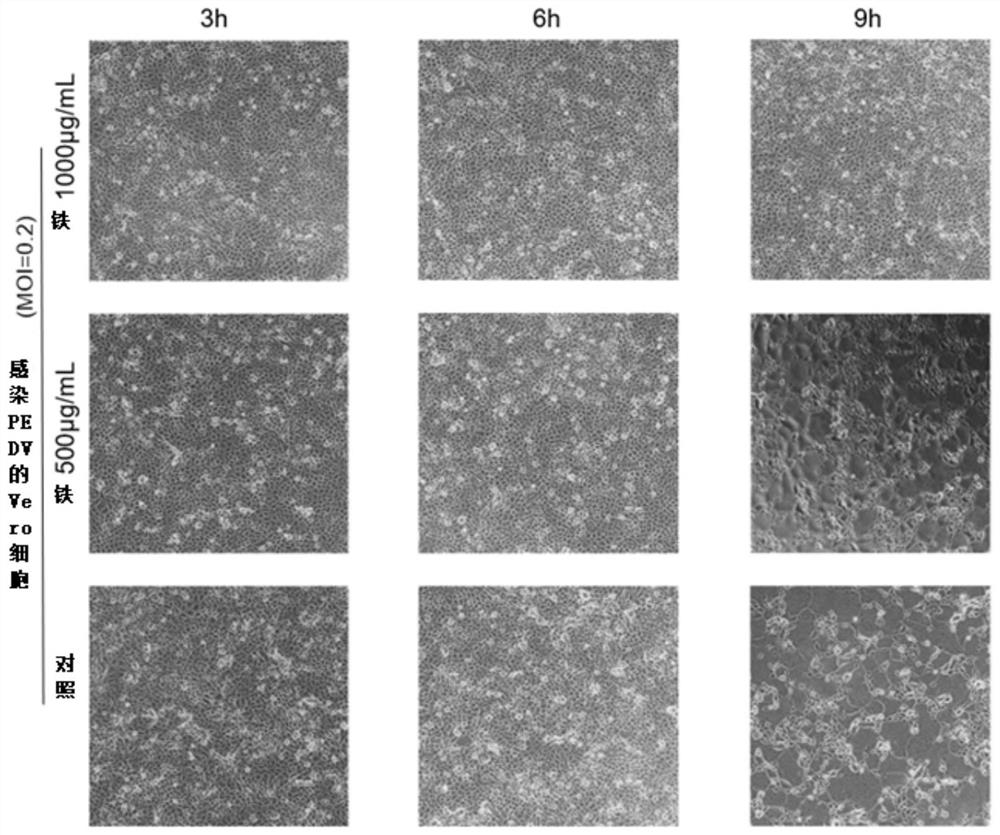
[0060] Example 1 Pelargonium extract inhibits PEDV test in vitro
[0061] 1.1 Toxicity test of geranium extract to Vero cells
[0062] Inoculate 6 × 10 in a 96-well plate 5 Cells / mL cell suspension (100 μL / well), leave a column without inoculating cells. At 37°C, 5% CO 2 cultured until the cells were fully confluent. The culture medium was aspirated, washed 3 times with PBS, and the geranium extract (hereinafter abbreviated as PE9) was diluted with DMEM to different concentrations, and added to a 96-well plate (100 μL / well), with 8 replicates for each dilution concentration. At 37°C, 5% CO 2 After culturing for 24h and 48h under certain conditions, the drug solution was sucked off, washed 3 times with PBS, and 10% CCK-8 solution was prepared in DMEM and added to a 96-well plate (100 μL / well). 2 Incubate for 1 h under the conditions, measure the absorbance (λ=450nm) with a microplate reader, and calculate the cell viability after 24 h and 48 h of treatment with different c...
Embodiment 2
[0084] Example 2 Fennel Extract Inhibits PEDV Proliferation in Vitro
[0085] 2.1 Toxicity test of fennel extract on Vero cells
[0086] The steps of the experimental method are as described in 1.1 of Example 1. The result is as Figure 4 As shown, through comparative experiments, when the concentration of fennel extract (abbreviated as A) is 1000 μg / mL or below, it has no significant effect on the viability of Vero cells.
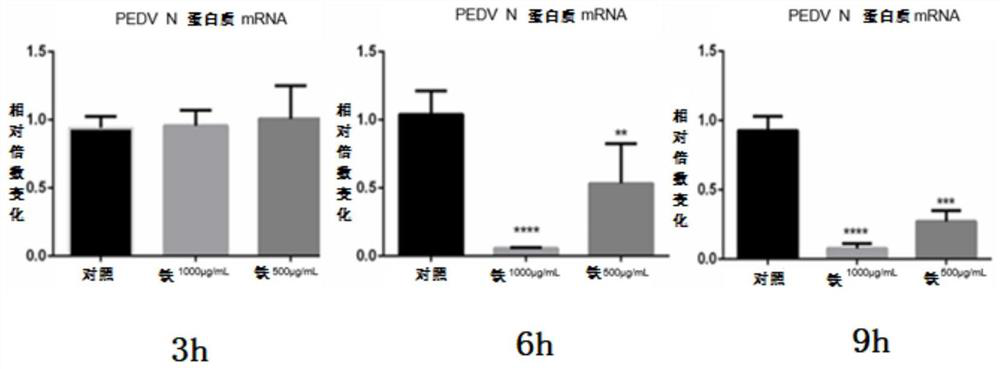
[0087] 2.2. The test of fennel extract inhibiting the proliferation of PEDV in Vero cells
[0088] The steps of the experimental method are as described in 1.2 of Example 1. In this example, the fennel extract (A) was compared with other medicines. Two concentration gradients were taken at the highest safe concentration of each drug for 24 hours, and the drug was added during the whole process of PEDV proliferation. At the end of the first replication cycle of PEDV, that is, at 6h.p.i., RNA samples were harvested, and real-time quantitative fluorescent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com