A kind of polysubstituted naphthoquinone derivatives and its preparation method and application
A derivative and multi-substituted technology, applied in the field of multi-substituted naphthoquinone derivatives and their preparation, can solve problems such as large toxic and side effects, and achieve the effects of low cost, good inhibitory effect and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 many substituted naphthoquinone derivatives
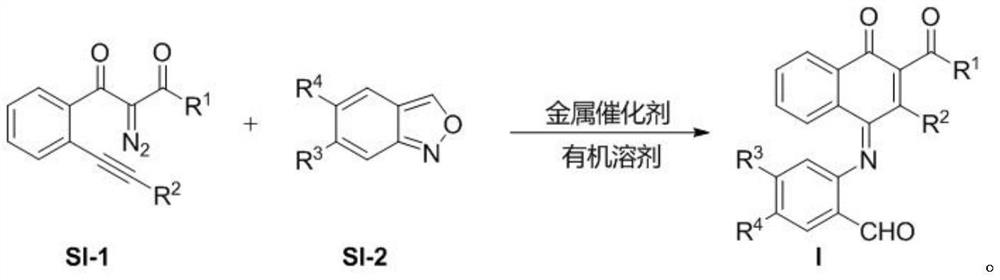
[0041] The preparation method of many substituted naphthoquinone derivatives is carried out according to the following reaction formula:
[0042]
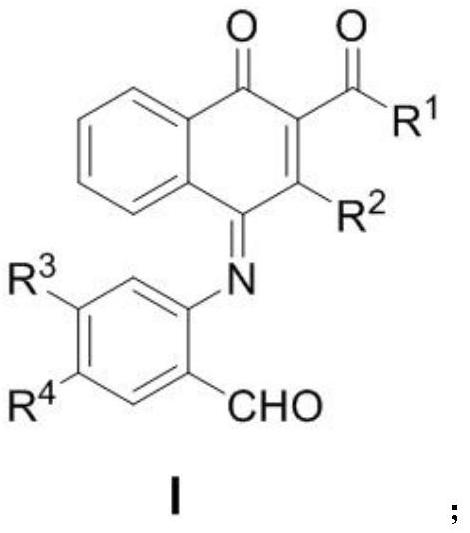
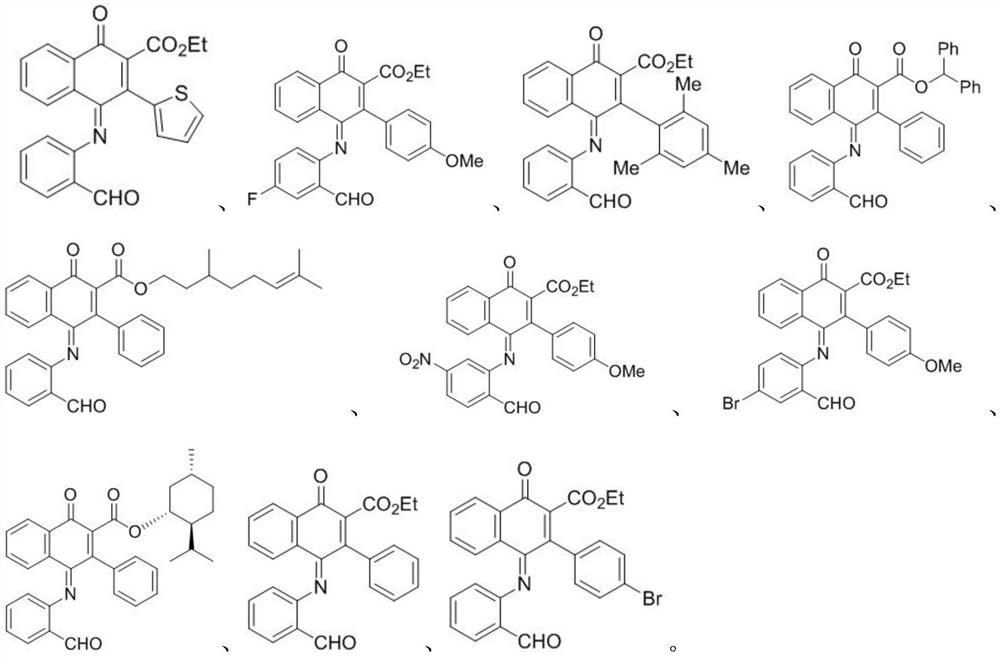
[0043] In the formula, R 1 Is ethoxy, diphenylmethoxy, Maolin alcohol, D-menthol, etc.; R 2 For thiophene, phenyl, p-bromophenyl, p-methoxyphenyl, 2,4,6-trimethylphenyl, etc.; R 3 For hydrogen, nitro, etc.; R 4 For hydrogen, fluorine, bromine, etc.
[0044]The preparation method of many substituted naphthoquinone derivatives is specifically: the diazo compound (0.20mmol) shown in formula SI-1 in the above-mentioned reaction formula, the benzisoxazole (0.24mmol) shown in formula SI-2 and catalyst ( Acetonitrile) [(2-biphenyl) di-tert-butylphosphine] gold (I) hexafluoroantimonate (0.01mmol) was weighed in a test tube, and then 2 mL of anhydrous 1,2-dichloroethane was added to the reaction system, The reaction was stirred at 30° C. for 24 hour...
Embodiment 2
[0059] Example 2 Inhibitory activity of multi-substituted naphthoquinone derivatives on colorectal adenocarcinoma cells
[0060] 1. The human colorectal adenocarcinoma cells used in the determination are: colorectal adenocarcinoma cells (HCT-116) (purchased from Guangzhou Saiku Biotechnology Co., Ltd.);
[0061] 2. Using the CCK-8 method to measure the inhibitory effect of polysubstituted naphthoquinone derivatives on the proliferation of human colorectal adenocarcinoma cells, wherein the specific determination process of the inhibitory rate of HCT-116 cells is as follows:
[0062] 1) Add 100 μL of cell suspension prepared with complete medium to the 96-well plate (5000 cells / well), and add 100 μL of cell culture solution without cells to the blank well as a control, and inoculate the well The 96-well culture plate was pre-incubated for 24 hours in an incubator (37°C, 5% CO 2 );
[0063] 2) Add 1.0 μL of a solution of the compounds to be tested (compounds I-1 to I-10) dissol...
Embodiment 3
[0079] Example 3 Inhibitory activity of multi-substituted naphthoquinone derivatives on osteosarcoma cells
[0080] 1. The human osteosarcoma cells used in the determination are: human osteosarcoma cells (SJSA-1) (purchased from Guangzhou Saiku Biotechnology Co., Ltd.);
[0081] 2. The inhibitory effect of multi-substituted naphthoquinone derivatives on human osteosarcoma cell proliferation was determined by CCK-8 method, wherein the specific determination process of the inhibition rate of SJSA-1 cells was as follows:
[0082] 1) Add 100 μL of cell suspension prepared with complete medium to the 96-well plate (5000 cells / well for inoculation), and add 100 μL of cell culture solution without cells to the blank well as a control, and inoculate the well The 96-well culture plate was pre-incubated for 24 hours in an incubator (37°C, 5% CO 2 );
[0083] 2) Add 1.0 μL of a solution of the compounds to be tested (compounds I-1 to I-10) dissolved in DMSO to the culture plate so that...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com