Preparation method of polymer of lipoic acid compound
A technology for lipoic acids and compounds, which is applied in the field of polymer preparation, can solve the problems of difficult control of configuration and molecular weight, complicated reaction conditions, etc., and achieves the effects of rapid and efficient ring-opening polymerization, simple preparation method and easy preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Solvent-free direct hot melt catalytic polymerization:
[0035]Weigh lipoic acid powder (3g, 0.0145mol) into a clean small flask, heat in an oil bath at 70°C for about 10 minutes until the powder melts, start stirring, then add 20uL 0.725mol / L N-butylbenzyl with a pipette Add the acetone solution of amine hexafluorophosphate to the flask and continue to heat and stir for 5 minutes. The molar ratio of the lipoic acid compound to the catalyst is 4000:4, the heating is stopped, and the liquid is cooled to room temperature, that is, the hot-melt catalytic polymerization process is completed, and the xerogel of the lipoic acid polymer is obtained.
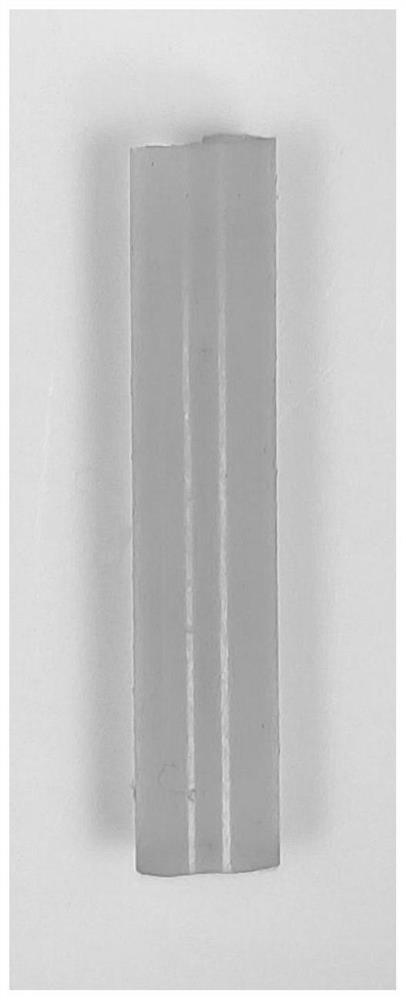
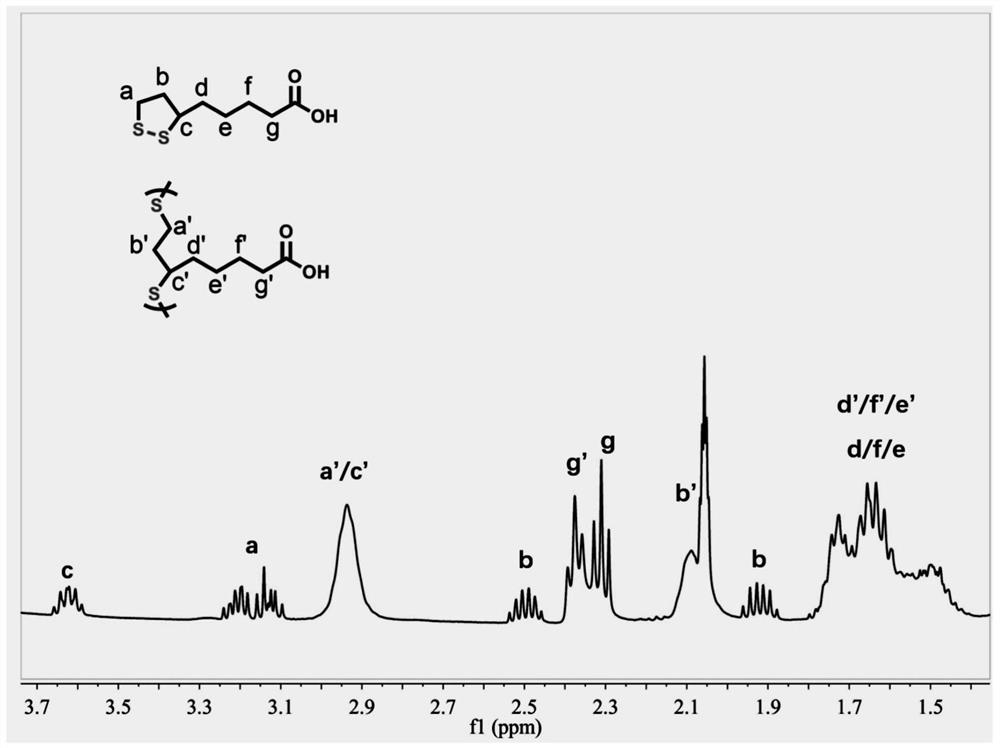
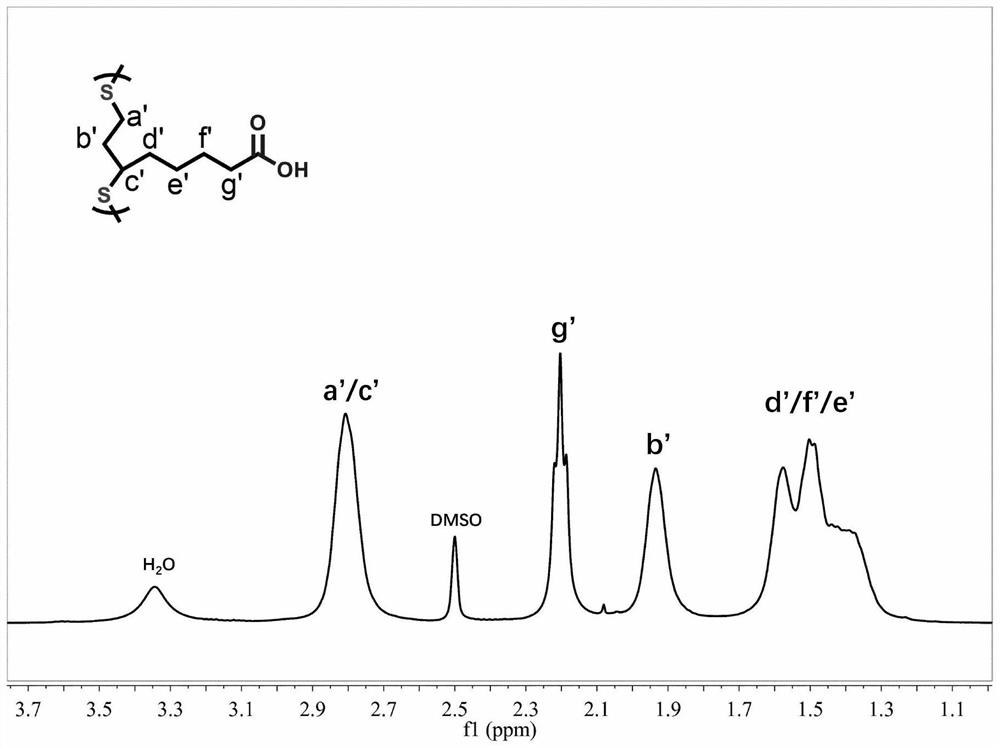
[0036] figure 1 It is a schematic diagram of the lipoic acid polymer image obtained by solvent-free direct hot-melt catalytic polymerization in Example 1, as can be seen from the figure, it is yellow transparent elastic xerogel; figure 2 It is the proton nuclear magnetic resonance spectrum of the lipoic acid polymer xerogel ob...
Embodiment 2~4
[0038] Except changing the mol ratio of lipoic acid added, catalyzer, all the other steps and conditions are identical with embodiment 1, specifically as shown in table 1:
[0039] Table 1
[0040]
Embodiment 5
[0042] Solution-catalyzed polymerization at room temperature: take lipoic acid powder (3g, 0.0145mol) and place it in a clean small flask, add 4.2ml of methylene chloride, the concentration of lipoic acid dissolved in methylene chloride is 3.5mol / L, stir and dissolve, Then use a pipette to add 20uL 0.725mol / L N-butylbenzylamine hexafluorophosphate acetone solution into the flask, continue to stir for 1 minute, the solution viscosity gradually increases, and then take it out and place it in an open polytetrafluoroethylene mold On the condition of room temperature, the solvent was volatilized for 1 hour to obtain xerogel of lipoic acid polymer.
[0043] Figure 4 It is a schematic diagram of the gel image of the lipoic acid polymer obtained by catalytic polymerization at room temperature in Example 5; it can be seen from the figure that it is in the form of a yellow transparent elastic xerogel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com