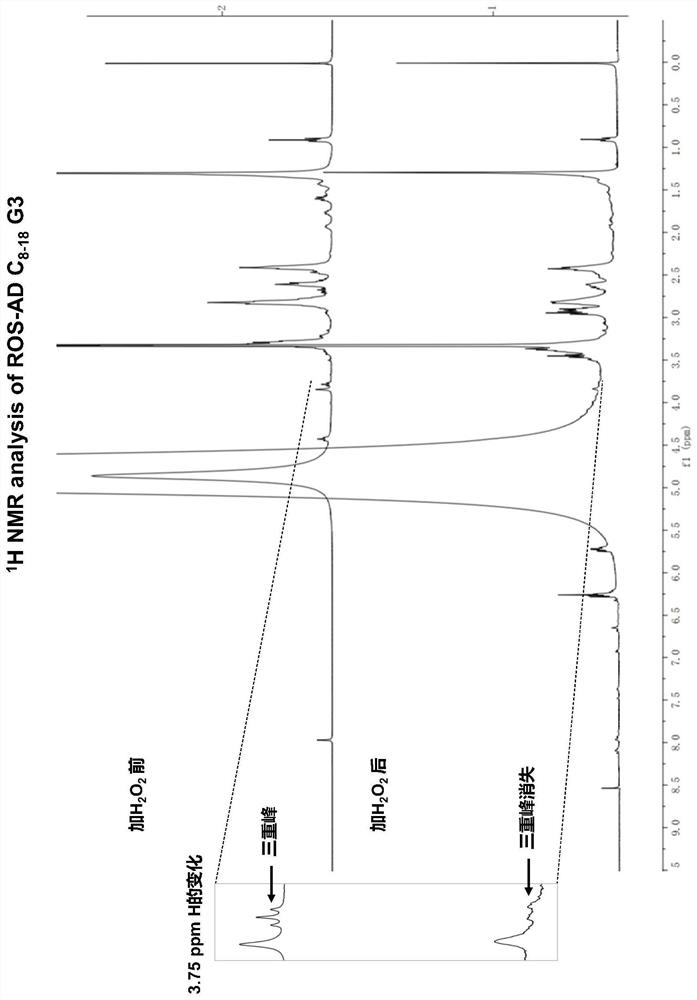
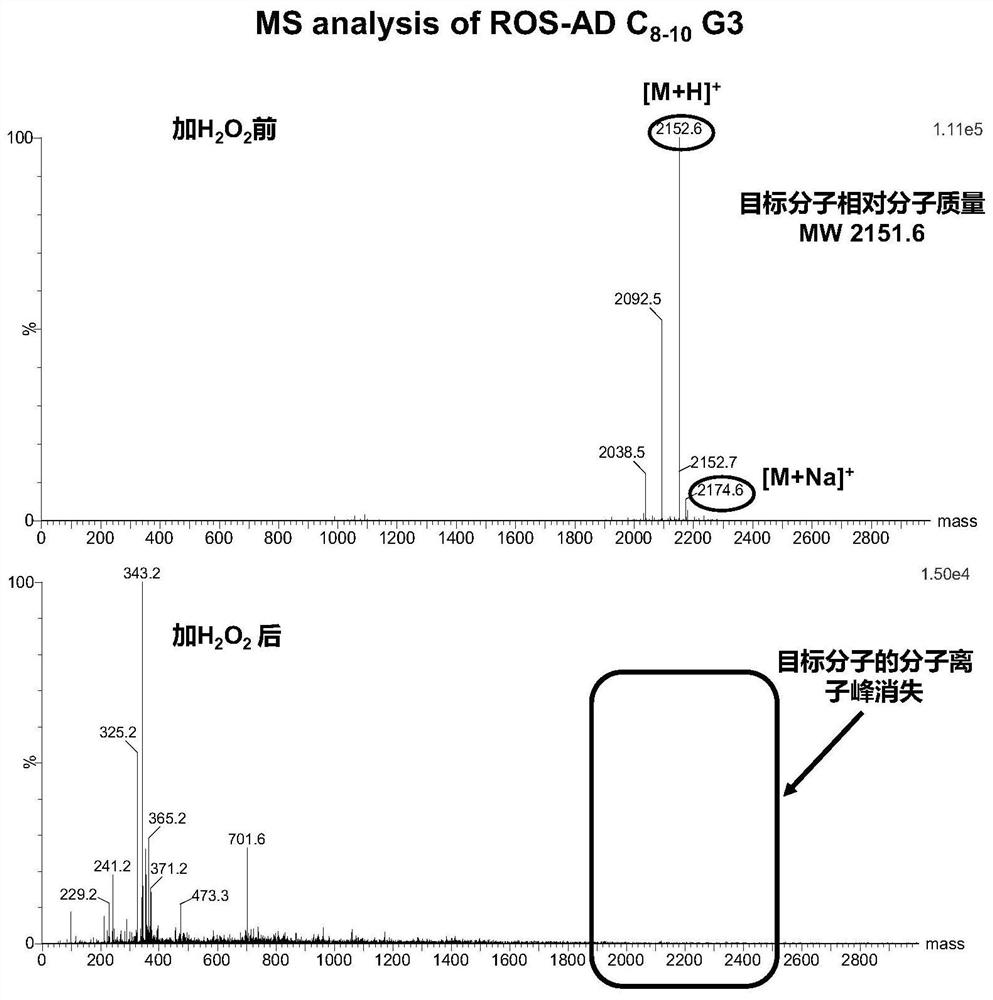
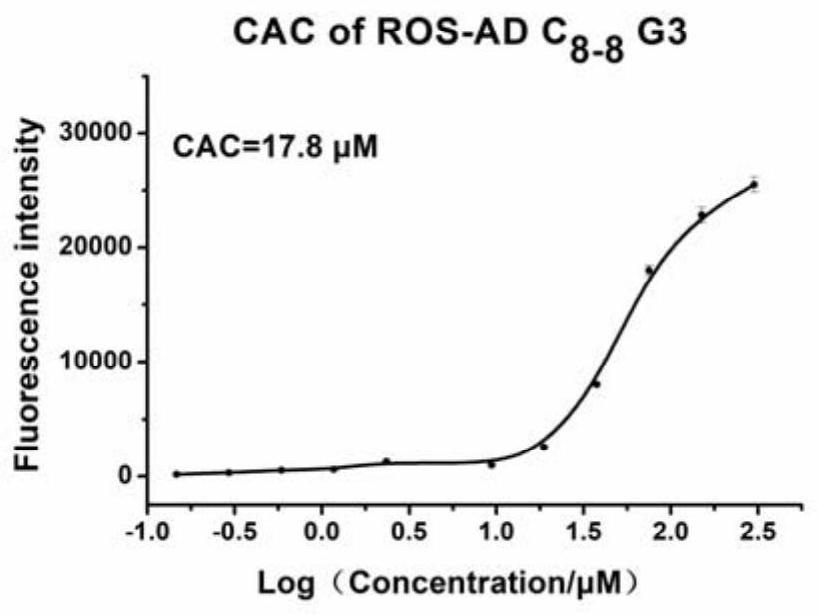
Amphiphilic dendrimer, synthesis method thereof, and application of amphiphilic dendrimer as drug delivery system
A technology of compound and alkyl, which is applied in the medical field and can solve the problems that the specificity and efficiency of nucleic acid or drug release need to be further improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Example 1: Compound ROS-AD C 8-10 Preparation of G3
[0097]
[0098]
[0099] 1.1 ROS-AD 1-1C 8 preparation of
[0100] Add 1,8-octanediol (585.2g, 4.0mmol), triethylamine (485.6mg, 4.8mmol) and DCM (8.0mL) in sequence in the reaction flask, slowly add TsCl (762.2mg, 4.0mmol) DCM (8.0 mL) solution was stirred at room temperature until the reaction was complete. After adding water, the liquid was separated, and then the aqueous phase was extracted with DCM, the organic phase was dried and filtered, the solvent was distilled off under reduced pressure, and the residue was separated by silica gel column chromatography to obtain a colorless oily liquid ROS-AD 1-1C 8 (895.3 mg, 75%).
[0101] 1 H NMR (300MHz, CDCl 3 ): δ7.81(d, J=8.3Hz, 2H), 7.37(d, J=7.9Hz, 2H), 4.04(t, J=6.5Hz, 2H), 3.65(t, J=6.6Hz, 2H ),2.47(s,3H),1.83–1.44(m,4H),1.43–1.16(m,9H).LC-MS(ESI,m / z):301.15[M+H] + .
[0102] 1.2 ROS-AD 1-2C 8 preparation of
[0103] Add NaHCO in turn to the re...
Embodiment 2
[0123] Example 2: Compound ROS-AD C 8-18 Preparation of G3
[0124]
[0125]
[0126] 2.1 ROS-AD 1-1C 8 preparation of
[0127] Same as 1.1 of 1 in the embodiment.
[0128] 2.2 ROS-AD 1-2C 8 preparation of
[0129] Same as 1.2 in Example 1.
[0130] 2.3 ROS-AD 1-3C 18 Preparation of Compound ROS-AD 1-3C by a similar method in Example 1.3 except that decyl bromide was replaced with 1-bromooctadecane 18 (95%).
[0131] 1 H NMR (300MHz, CDCl 3 )δ=2.86(t,J=7.5Hz,2H),2.32(s,3H),1.63–1.49(m,2H),1.40–1.18(m,30H),0.87(t,J=6.6Hz,3H ).
[0132] 2.4 ROS-AD 1-4C 18 preparation of
[0133] Except with ROS-AD 1-3C 18 Replace ROS-AD 1-3C 10 In addition, compound ROS-AD 1-4C was prepared in a similar manner in Example 1.4 18 (100%).
[0134] 1 H NMR (300MHz, CDCl 3 )δ=2.52(q,J=7.1Hz,2H),1.63–1.49(m,2H),1.44–0.97(m,30H),0.88(t,J=6.6Hz,3H).
[0135] 2.5 ROS-AD 1-5C 8-18 preparation of
[0136] Except with ROS-AD 1-4C 18 Replaces ROS-AD 1-4C 10 In addition, compoun...
Embodiment 3
[0147] Example 3: Compound ROS-AD C 8-8 Preparation of G3
[0148]
[0149]
[0150] 3.1 ROS-AD 1-1C 8 preparation of
[0151] Same as 1.1 in Example 1.
[0152] 3.2 ROS-AD 1-2C 8 preparation of
[0153] Same as 1.2 in Example 1.
[0154] 3.3 ROS-AD 1-3C 8 preparation of
[0155] Except replacing decyl bromide with n-octane bromide, compound ROS-AD1-3C was prepared in a similar manner in Example 1.3 8 (95%).
[0156] 1 H NMR (300MHz, CDCl 3 )δ=2.86(t,J=7.5Hz,2H),2.32(s,3H),1.64–1.47(m,2H),1.46–0.97(m,10H),0.87(t,J=6.6Hz,3H ).
[0157] 3.4 ROS-AD 1-4C 8 preparation of
[0158] Except with ROS-AD 1-3C 8 Replace ROS-AD 1-3C 10 In addition, compound ROS-AD 1-4C was prepared in a similar manner in Example 1.4 8 (100%).
[0159] 3.5 ROS-AD 1-5C 8-8 preparation of
[0160] Except with ROS-AD 1-4C 8 Replaces ROS-AD 1-4C 10 In addition, compound ROS-AD 1-5C was prepared according to a similar method in Example 1.5 8-8 (65%).
[0161] 1 H NMR (300MHz, CDC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com