Preparation method of vitamin A acetate
A technology of acetate and vitamin, which is applied in the field of preparation of vitamin A acetate, can solve the problems of slow elimination and decomposition reaction of intermediates, difficult post-processing, poor selectivity, etc., and achieve long-term stable storage, oxidation resistance and thermal stability. The effect of good stability and good market competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0077] In the preparation method of vitamin A acetate of the present invention, reaction formula is as follows:
[0078]
Embodiment 1
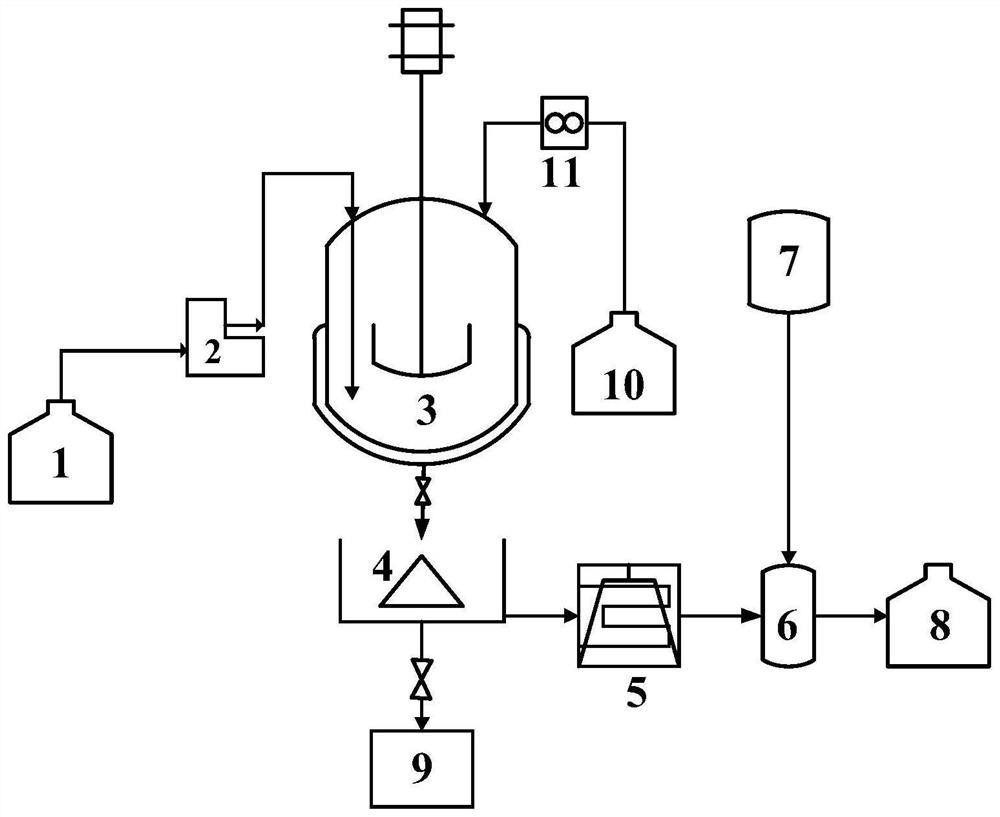
[0081] A kind of preparation method of vitamin A acetate, process flow chart is as figure 1 As shown, the specific steps are as follows:
[0082] (1) Add 106g sodium carbonate solid powder in the first raw material tank 1, add 1060mL water under stirring state, mix and obtain material B; Add 501.08g C15 phosphine salt, 142g C5 aldehyde in the second raw material tank 10, stir state Add 2500mL of mixed solvent (the volume ratio of water, methanol and n-hexane is 100:1:10), mix well to obtain material A, and nitrogen blanket for use;
[0083] (2) Reactor 3 is replaced with nitrogen 5 times before the reaction, adjust the rotation speed of the stirring paddle in the reactor to 100rpm, raise the temperature of the reactor to 25°C, and adjust the system pressure to 0.1MPaG, then control the first flowmeter 2 and the second Two flow meters 11, slowly drop material B and material A into the reactor simultaneously, the space velocity of material B and material A are both 14h -1 , ke...
Embodiment 2
[0088] A kind of preparation method of vitamin A acetate, process flow chart is as figure 1 As shown, the specific steps are as follows:
[0089] (1) Add 138g potassium carbonate solid powder in the first raw material tank 1, add 280mL water under stirring state, mix and obtain material B; Add 501.08g C15 phosphine salt, 156.2g C5 aldehyde in the second raw material tank 10, stir Add 2500mL mixed solvent (the volume ratio of water, methanol and n-hexane is 200:1:5) under the state, mix well to obtain material A, and nitrogen blanket for use;
[0090] (2) Reactor 3 is replaced with nitrogen 5 times before the reaction, adjust the rotating speed of the stirring paddle in the reactor to 300rpm, raise the temperature of the reactor to 25°C, and adjust the system pressure to 1.0MPaG, then control the first flowmeter 2 and the second Two flow meters 11, slowly drop material B and material A into the reactor simultaneously, the space velocity of material B and material A are both 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com