Attenuated virus of flavivirus and application of attenuated virus
A yellow fever virus, virus technology, applied in the direction of virus, antiviral agent, inactivation/attenuation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Embodiment 1: Construction and virus rescue of flavivirus attenuated strain clone
[0115] 1. Cloning construction of flavivirus attenuated strain
[0116] Schematic diagram of the cloning construction of attenuated flavivirus strains figure 1 shown.
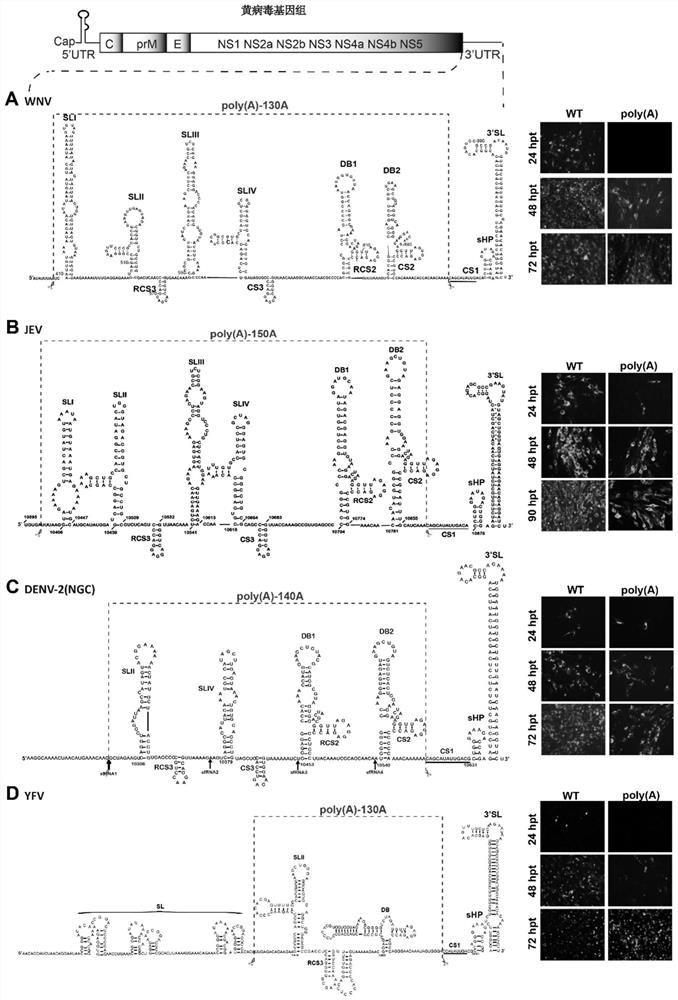
[0117] Using the plasmid DNA of the infectious clone of the wild-type (WT) West Nile virus (WNV) strain as the backbone, the sequence from SLI to CS2 (including the 5' Terminal stem-loop region I (SLI), stem-loop region II (SLII), repeat circularization sequence region 3 (RCS3), stem-loop region III (SLIII), stem-loop region IV (SLIV), circularization sequence region 3 (CS3 ), dumbbell region 1 (DB1), repeat circularizing sequence region 2 (RCS2), dumbbell region (DB2), circularizing sequence region 2 (CS2)) deletion, retaining circularizing sequence region 1 (CS1) in the 3' untranslated region ) and the short hairpin-3' stem-loop region (sHP-3'SL) after circularizing sequence region 1, and inserting a poly(A) sequence...
Embodiment 2
[0132] Construction and virus rescue of other West Nile virus attenuated strain clones of embodiment 2
[0133] 1. Construction of clones of other West Nile virus attenuated strains
[0134] Schematic diagram of the clone construction of other West Nile virus attenuated strains as shown in figure 2 shown.
[0135] Using the plasmid DNA of the infectious clone of the West Nile virus (WNV) strain of the wild type (WT) in Example 1 as a backbone, the sequence from SLI to CS3 in the 3' untranslated region (3'UTR) of WNV (including SLI, SLII, RCS3, SLIII, SLIV, CS3) deleted, retaining dumbbell region 1 (DB1), repeat circularization sequence region 2 (RCS2), dumbbell region 2 (DB2), circularization sequence in the 3' untranslated region Region 2 (CS2), Circularization Sequence 1 (CS1), and the short hairpin-3' stem-loop region after cyclization Sequence 1 (sHP-3'SL), with deletion of the sequence from SLI to CS3 The poly(A) sequence (130 adenosine nucleotides) was inserted into ...
Embodiment 3
[0144] Embodiment 3: Flavivirus attenuated strain and wild-type virus growth curve comparison
[0145] 1. Virus Titer Determination
[0146] The titer of the virus was determined by the plaque method, and the specific steps were as follows:
[0147] Inoculate 1×10 cells in each well of a 24-well cell culture plate 5 For each BHK-21 cell, when the cell confluency reaches 90%, discard the medium in the well, and add 100 μl of the poly of the attenuated flavivirus collected in Step 3 of Example 1 diluted 10 times with DMEM medium containing 2% FBS. (A) P0 generation virus, adsorb for 1 hour at 37°C, and shake fully every 15 minutes. After the adsorption is complete, the virus liquid in each well is discarded, and the DMEM medium containing 2% FBS and the cover of 2% methylcellulose are added, and the virus solution is incubated at 37°C and 5% CO. 2 Cultured in an incubator for 3 days, stained with staining solution containing 1% crystal violet and 3.7% formaldehyde after plaqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com