Synthesis method and application of vidarabine monophosphate
An adenosine monophosphate arabinoside and a synthesis method technology, applied in chemical instruments and methods, sugar derivatives, sugar derivatives and other directions, can solve problems such as unfavorable industrial production, improve the overall reaction yield, simplify synthesis steps, The effect of simplifying industrial production steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
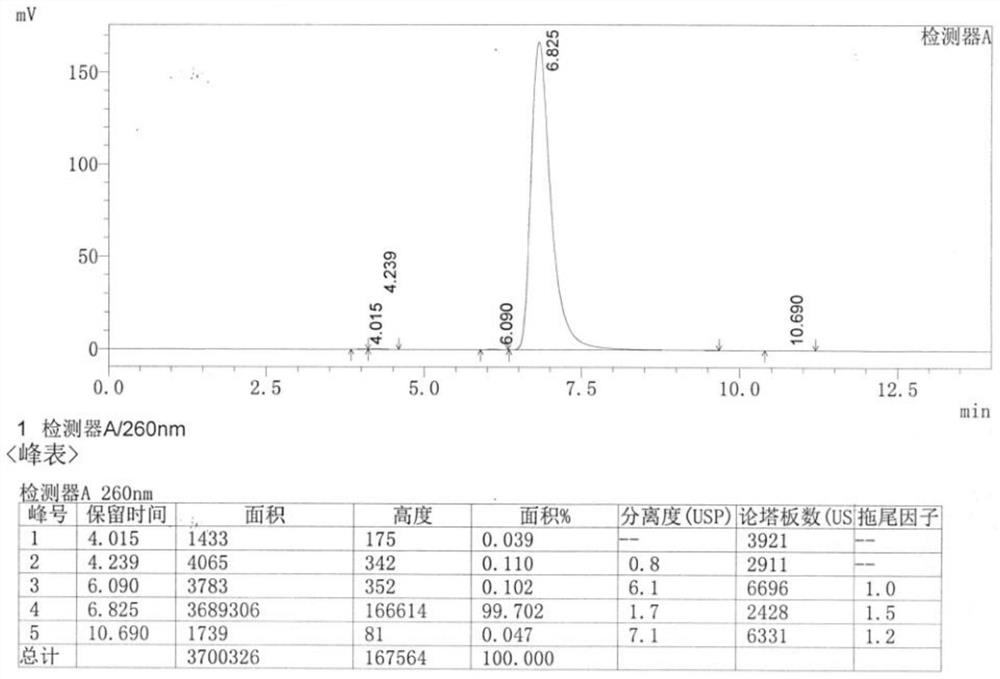
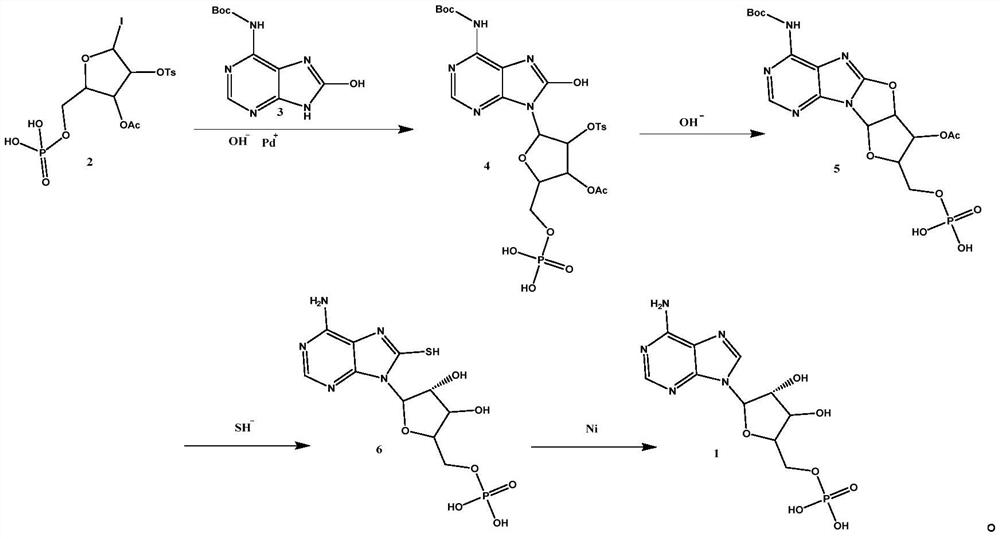
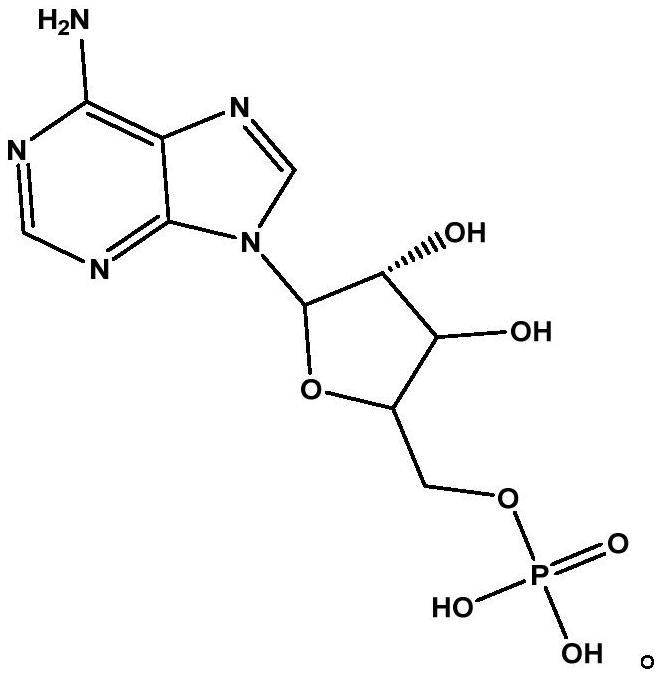
Image
Examples
Embodiment 1
[0038] Embodiment 1 A kind of synthetic method of vidarabine monophosphate M1
[0039] This embodiment provides a synthetic method of adenosine vidarabine monophosphate M1, the synthetic method comprising the following steps:
[0040] 1) Weigh 53.6g 5-iodo-2-((phosphocarboxy)methyl)-4-(tosyloxy)tetrahydrofuran-3-yl acetate (2) and 30g tert-butyl ( 8-Hydroxy-9H-purin-6-yl) carbamate (3) was mixed in 220ml THF, 31.8g sodium carbonate and 5.78g tetrakis(triphenylphosphine)palladium were added at 70°C, and the condensation reaction was carried out for 8h, The progress of the reaction was monitored by TLC, and the reaction was terminated after the ultraviolet color point of the raw material (2) disappeared. The reaction solution was filtered, the filter cake was washed three times with ethanol, the filtrate was collected and concentrated under reduced pressure until no solvent was evaporated to obtain 39.54g compound (4) crude product (60% yield), which was directly used in the ne...
Embodiment 2~6
[0049] The synthetic method of embodiment 2~6 adenosine monophosphate M2~M6
[0050] Examples 2-6 provide the synthesis method of vidarabine monophosphate M2-M6, the synthesis method is roughly the same as the synthesis method of vidarabine monophosphate provided in Example 1, the only difference is that some synthesis process parameters are different , and the specific synthesis process parameters are shown in Table 1.
[0051] Table 1: Synthetic process parameters of vidarabine monophosphate M2-M6
[0052]
[0053]
[0054] All the other processing parameters are identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com