Construction method of organic phase electrochemical luminescence system of rigid spiro silole compound
A construction method and an organic phase technology, applied in organic chemistry, chemical instruments and methods, silicon organic compounds, etc., can solve problems such as the luminous efficiency of silole compounds, and achieve improved luminous efficiency and sensitivity, good stability and Reproducible, low-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for synthesizing a rigid spirocyclic silole compound and a method for constructing an electrochemiluminescent system, the steps of which are as follows:
[0027] a, the preparation method of rigid spirocyclic silole compound 5,5'-spirobis[dibenzo[b,d]silole]
[0028] (1) Under the protection of an inert gas, add 2.00g of 2,2-dibromobiphenyl to a 100mL Schlenk bottle, then add 35mL of ultra-dry tetrahydrofuran, and then dissolve under magnetic stirring until the 2,2-dibromobiphenyl is completely After dissolving, add 6.16mL of n-butyl lithium to the Schlenk bottle, react at -50°C for 1h, then warm to room temperature, and continue stirring for 12h; then, add 0.546g of distilled silicon tetrachloride dropwise at 0°C And stirred for 1h, then warmed to room temperature, and continued to stir for 24h;
[0029] (2) After the reaction, transfer the solution to a round-bottomed flask for rotary evaporation, then add 20 mL of dichloromethane to dissolve, filter out ins...
Embodiment 2
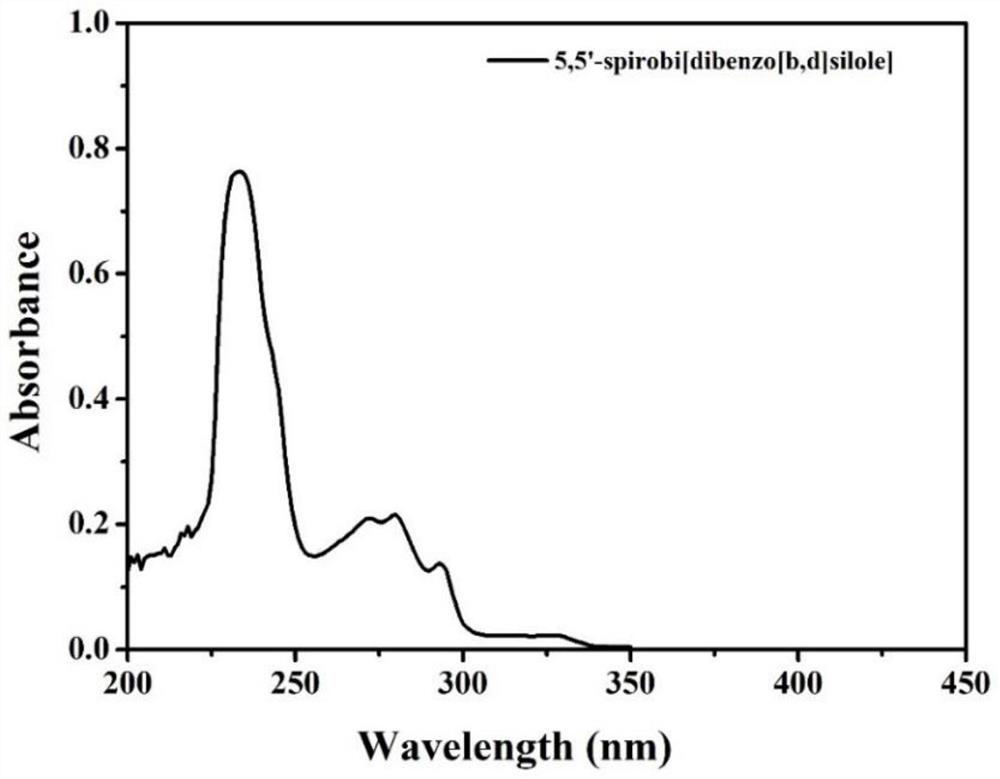
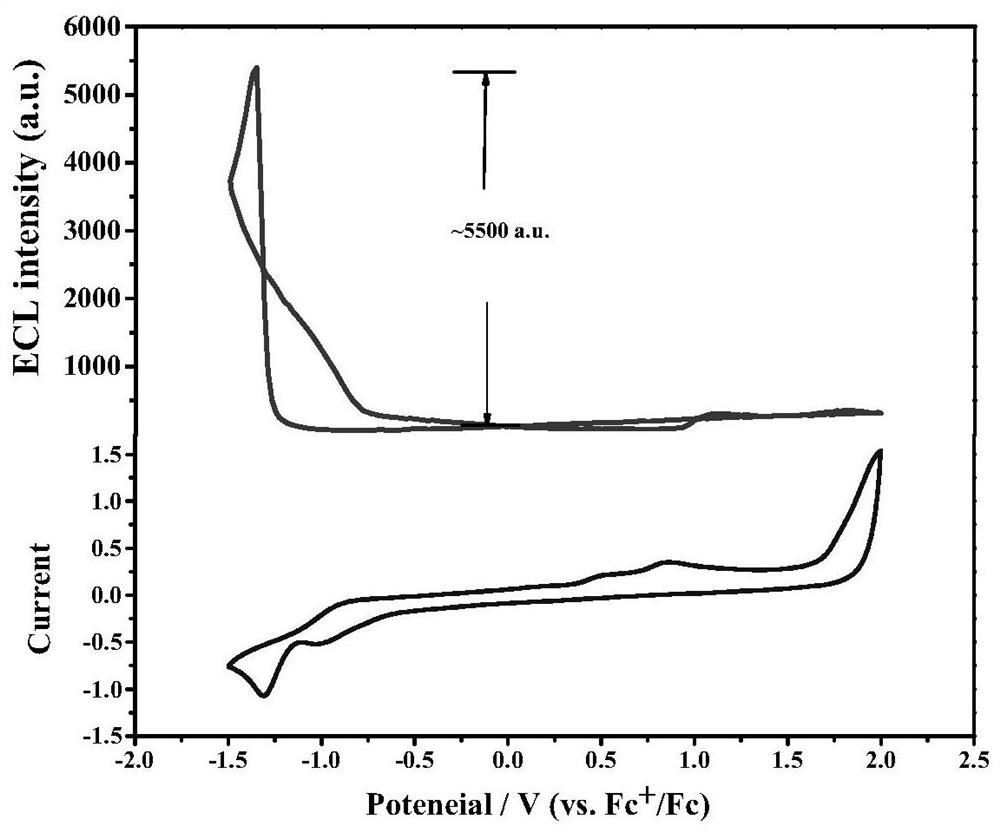
[0040] 1. Determination of the CV and ECL signal intensity-potential diagrams of 5,5'-spirobis[dibenzo[b,d]silole] in the supporting electrolyte tetrabutylammonium hexafluorophosphate system. The construction method of the system is the same as in Example 1, the concentration of the supporting electrolyte tetrabutylammonium hexafluorophosphate in the system constructed by the method is 0.1mol / L, and the potential scanning rate is 0.2V s -1 , The potential window is -1.5 ~ -2.0V. see results image 3 . The results showed that the ECL intensity of 5,5'-spirobis[dibenzo[b,d]silole] was about 5500.
[0041] 2. Optimize the potential window, scan rate, supporting electrolyte concentration and other conditions of the electrochemiluminescence system of 5,5'-spirobi[dibenzo[b,d]silole], and conduct ECL stability test. The construction method of the system is similar to Example 1. see results Figure 4 . The results show that the best potential window is -1.5~-2.0V, and the best ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com