Preparation method of vidarabine monophosphate freeze-dried powder injection for injection
A technology of adenosine vidarabine monophosphate freeze-dried powder and adenosine monophosphate, which can be used in freeze-dried transportation, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of drug stability And the appearance, shape and loose shape need to be improved, so as to achieve the effect of good appearance, guarantee of uniformity and reduction of impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 Stability experiment of vidarabine monophosphate at different pH values
[0033] Take 13 parts of 9.5 mL of freshly boiled and cooled distilled water, numbered 1 to 13 respectively, and use hydrochloric acid or sodium hydroxide to adjust the pH value to 4, 6, 7, 8, 9, 9.2, 9.3, 9.4, 10, 10.4, 10.5, 10.6, 11, add water to 10mL again;
[0034] Add 0.1g of vidarabine monophosphate to No. 1-13 water respectively, shake and mix well, let stand for 10min, and follow the "High Performance Liquid Chromatography Determination of the Content of Vidarabine Monophosphate" (Modern Hospital, 2007) April, Volume 7 Supplement, Pages 28-29) published detection method, using high performance liquid chromatography to measure the content of vidarabine monophosphate, the results show that when the pH value is 9.3-10.5, the content of vidarabine monophosphate is relatively stable If the pH value exceeds this range, it will affect the content of adenosine monophosphate. When the...
Embodiment 2
[0037] Example 2 A preparation method of adenosine vidarabine monophosphate freeze-dried powder for injection
[0038] 1) Refining of vidarabine monophosphate
[0039] Under the protection of nitrogen, take 2000mL distilled water, add 20wt% sodium hydroxide aqueous solution dropwise to adjust the pH value to 10.0, add 200g of vidarabine monophosphate crude product at room temperature and stir to dissolve, then add 5g of activated carbon, and stir at room temperature for 20min to decolorize , filtered, the resulting filtrate was adjusted to pH 7.0 with 10wt% dilute hydrochloric acid aqueous solution, cooled to -5°C, slowly added ethanol dropwise until a small amount of crystals precipitated, then stopped adding, kept at -5°C and stirred slowly for 6 hours, and the crystallization was completed Afterwards, it was filtered at low temperature, washed three times with 5 mL of ice water, and dried in vacuum at room temperature for 3 hours to obtain 185.4 g of vidarabine monophosphat...
Embodiment 3~7
[0059] Example 3-7 Preparation method of adenosine vidarabine monophosphate freeze-dried powder for injection
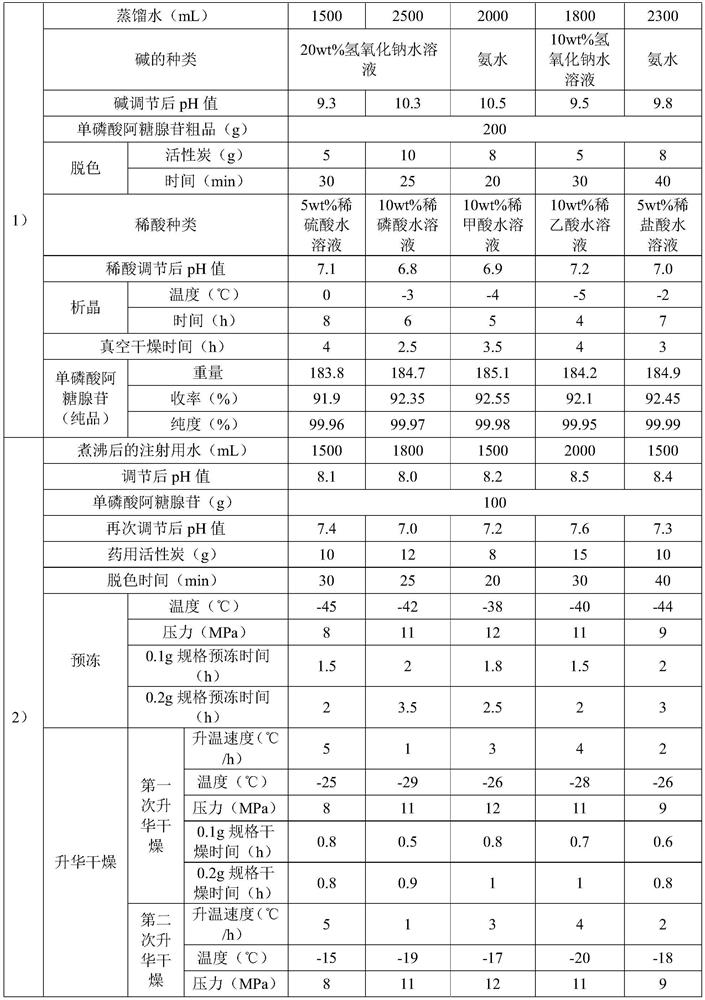
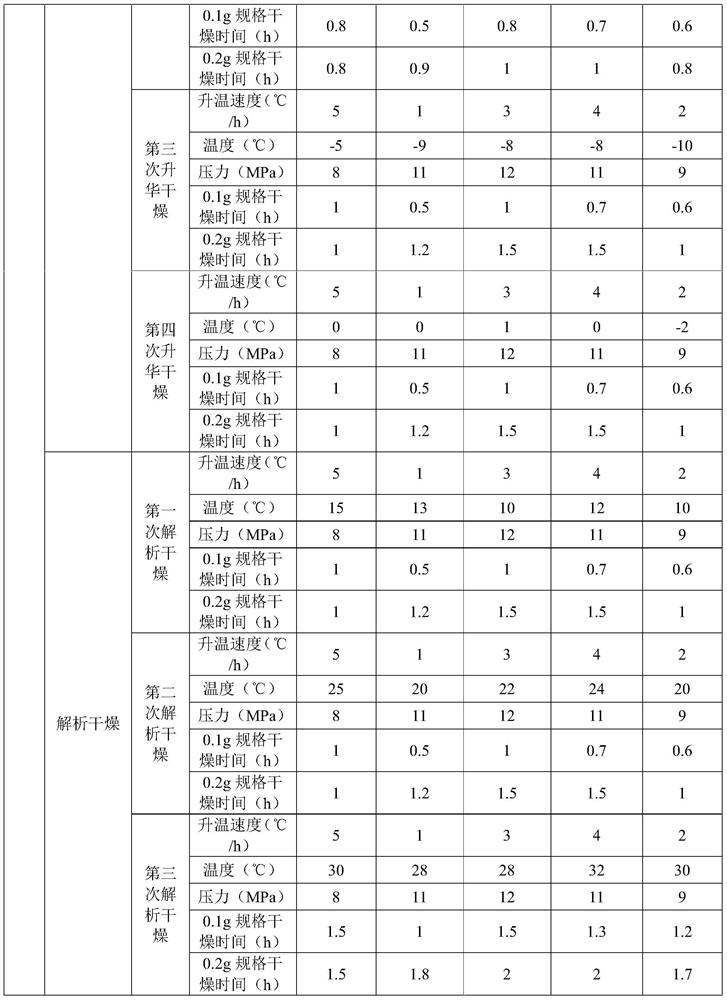
[0060] Examples 3 to 7 are respectively a preparation method of adenosine vidarabine monophosphate freeze-dried powder for injection, and their steps are basically the same as those in Example 2, except that the amount of raw materials and process parameters are different. See Table 3:
[0061] List of each process parameter in table 3 embodiment 3~7
[0062]
[0063]
[0064]
[0065] The contents of the other parts of Embodiments 3 to 7 are the same as those of Embodiment 2.
[0066] The adenosine vidarabine monophosphate freeze-dried powder for injection prepared in Examples 2-7 has good stability and clarity, high main drug content, less impurities, smooth surface, good appearance and excellent resolubility.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com