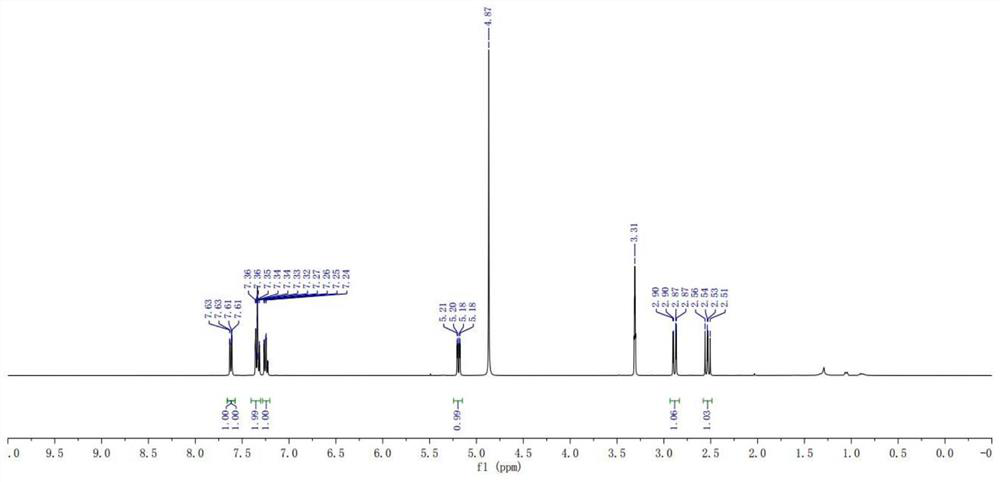
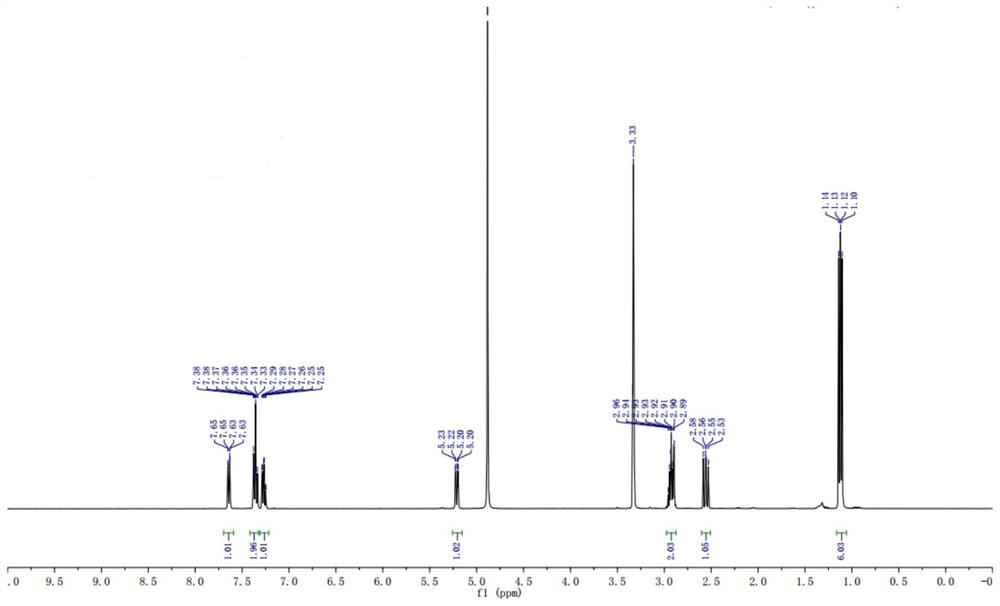
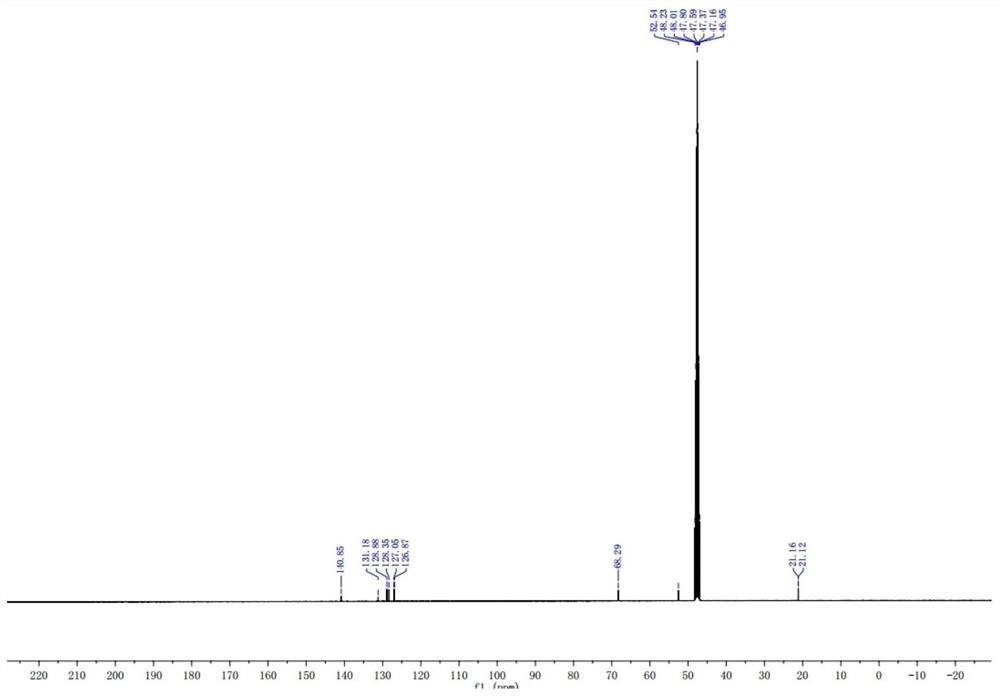
Preparation method of stable isotope-labeled clorprenaline
A technology of stable isotope and clorprenaline, which is applied in the field of preparation of clorprenaline, can solve the problems of not being suitable for large-scale preparation, difficult separation and purification of intermediates, high synthesis cost, etc., and achieves cheap raw materials and reproducibility And the effect of high stability and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of Intermediate II
[0038]
[0039] The raw material 2-bromo-2'-chlorophenylideophenone (compound I) (10.0 g) was dissolved in 100 mL of acetonitrile, dimethyla aminyl sodium (1.2 eq, 7.0 g) was added, and the reaction mixture was stirred at 70 degrees Celsius. 3h. TLC detection (PE; EA = 5: 1) The reaction was complete, and the reaction was also cooled and filtered, concentrated the mother liquor to give a crude product. The crude product was purified by flash column chromatography (eluent: ethyl acetate / petroleum ether = 0% to 30%) to give 9.0 g of intermediate II, yield 92.8%, yellow solid.
Embodiment 2
[0040] Example 2: Synthesis of Intermediate III
[0041]
[0042] 8 g of intermediate II was suspended with 6N HCl (100 mL) aqueous solution, and heated in an oil bath placed in an oil bath at 120 degrees Celsius, and then said, TLC detection (PE: EA = 3: 1) The reaction was complete. Static cooling overnight, precipitated solid, filtrate, dried to give 7.0 g of intermediate III, yield 96.0%, white solid.
Embodiment 3
[0043] Example 3: Synthesis of Intermediate IV
[0044]
[0045]The 7.2 g of intermediate III was dissolved in methanol (100 mL), and sodium borohydride was added at room temperature (ice bath), and sodium borohydride (3.0 Eq, 5.8 g) was added to obtain suspension. The reaction was reacted at room temperature. Add water to the reaction, add a small amount of potassium carbonate to 10 to 10, then extract about 3 times with dichloromethane, washed with saturated brine, dry, concentrate to obtain approximately 5.5 g intermediate IV, yield 91.7%, yellow solid .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com