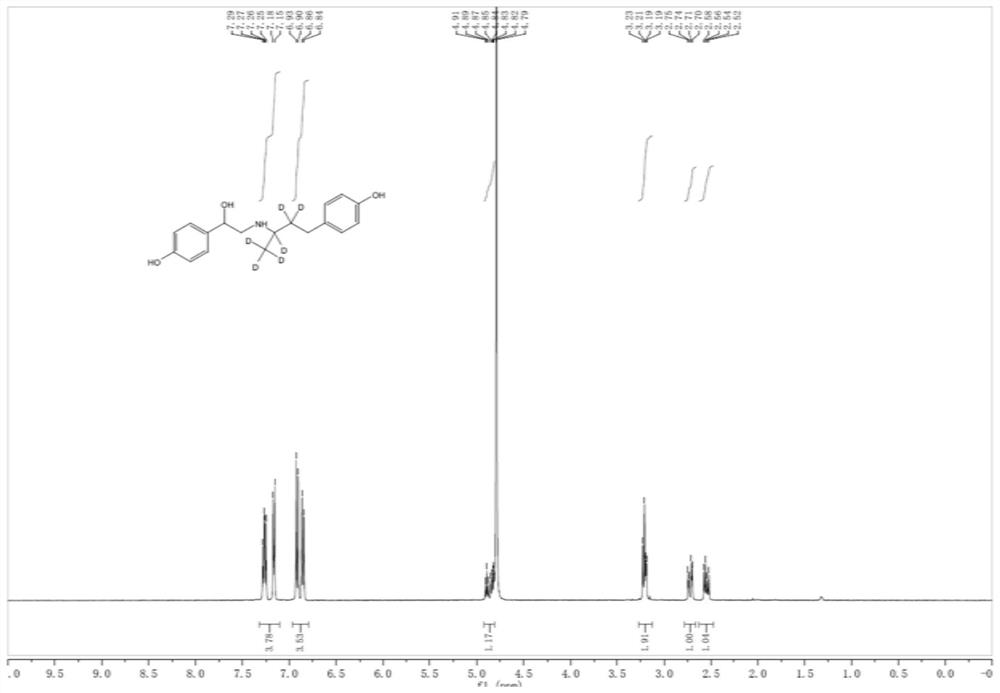
Preparation method of ractopamine-D6 hydrochloride
A technology of ractopamine hydrochloride and salt-forming reaction is applied in the field of drug synthesis, which can solve the problems of insufficient labeled compounds, expensive labeled raw materials, low yield and the like, and achieves the effects of low price, low synthesis cost and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
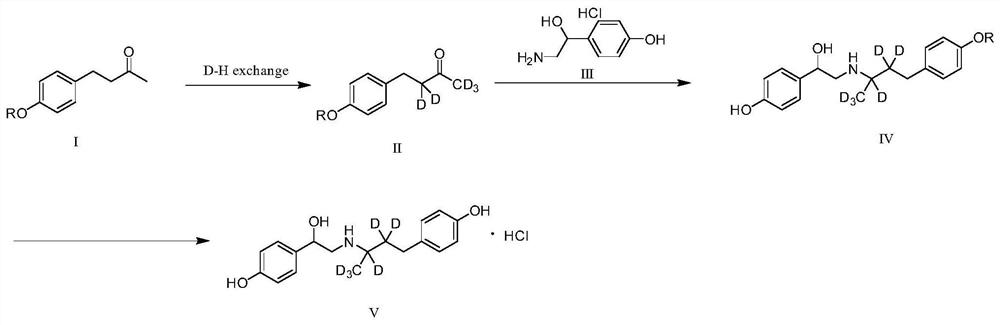
[0030] (1) Dissolve compound I (5.0g, 1.0eq) in dry 1,4-dioxane (50mL) at room temperature, under nitrogen protection, add 40% NaOD (17.3g, 6.0eq) heavy aqueous solution under stirring , then add D 2 O (100 mL), the temperature was raised to 50°C and the reaction was stirred overnight. The reaction solution was adjusted to pH=6 with deuterium hydrochloric acid aqueous solution, then extracted with dry methyl tert-butyl ether, the organic phases were combined, dried, and concentrated to obtain 5 g of compound IIa with a yield of 95%, which was directly used for Next step, the structural formula of compound IIa is:
[0031] (2) At room temperature, dissolve compound IIa (4g, 1.0eq) in CH 3OD (50mL), then add intermediate III (4.14g, 1.0eq), acetic acid (0.26g, 0.2eq), and cool to 0°C under nitrogen protection, at this temperature, add sodium cyanoborodeuteride in batches ( 2.2g, 1.5eq), returned to room temperature and stirred overnight. The reaction was monitored by TLC (...
Embodiment 2
[0035] (1) Dissolve compound I (5.0g, 1.0eq) in 50mL of dry tetrahydrofuran at room temperature, under nitrogen protection, add 40% NaOD (12.1g, 6.0eq) heavy aqueous solution under stirring, then add D 2 O (100 mL), heated to 50°C and stirred overnight. Adjust the pH of the reaction solution to about 6 with deuterium hydrochloric acid solution, then extract it with dry methyl tert-butyl ether, combine the organic phases, dry, and concentrate to obtain 5 g of compound IIb. The yield is 95%, and it is directly used for Next step, the structural formula of compound IIb is:
[0036] (2) at room temperature, compound IIb (4g, 1.0eq) was dissolved in 50mL of CH 3 In OD, add compound III (2.9g, 1.0eq), acetic acid (0.19, 0.2eq), and cool to 0°C under nitrogen protection. At this temperature, add sodium cyanoborodeuteride (1.5g, 1.5eq ), returned to room temperature and stirred overnight, and TLC (DCM:MeOH=10:1) monitored the reaction. After the reaction, the reaction solution wa...
Embodiment 3
[0039] The difference with embodiment 1 is that the specific operation of step (1) is:
[0040] Compound Ia (5.0 g, 1.0 eq) was dissolved in dry CH at room temperature 3 OD (50 mL), under nitrogen protection, sodium methoxide (4.6 g, 3.0 eq) was added with stirring, and the temperature was raised to 50° C. and the reaction was stirred overnight. The reaction solution was adjusted to pH=6 with deuterium hydrochloric acid aqueous solution, and then extracted with dry methyl tert-butyl ether, the organic phases were combined, dried, and concentrated to obtain 4.8g of compound IIa with a yield of 94%, which was directly used without purification in the next step.
[0041] Step (2) and step (3) are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com