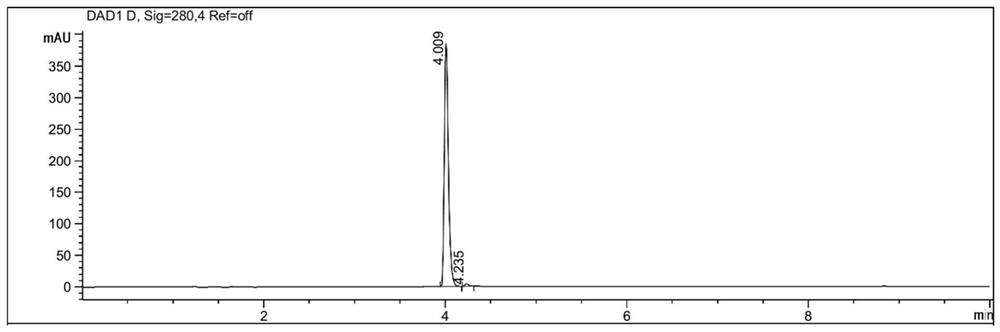
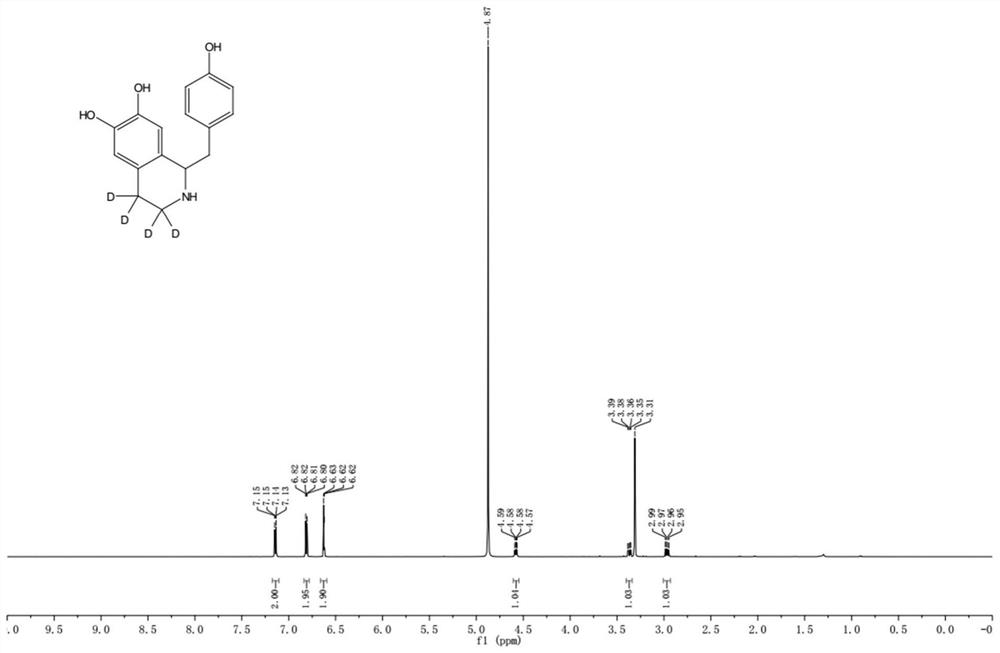
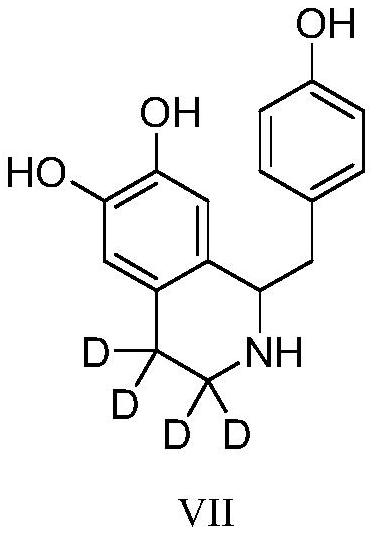
Preparation method of deuterium-labeled higenamine stable isotope compound
A technology of higenamine and its compound, which is applied in the field of isotope internal standard preparation, can solve problems such as unsatisfactory requirements and unsuitability, and achieve the effects of cheap raw materials, cost saving, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the synthesis of 3,4-dimethoxyphenylacetonitrile-D2 (IIa):
[0042]
[0043] The raw material 3,4-dimethoxyphenylacetonitrile (Ia) (10.0g) was dissolved in 100mL of dry THF, cooled to about -78°C, and n-BuLi (2.5M, 55mL, 2.3 eq), after adding, react at this temperature for about 1 hour. The reaction mixture was quenched with about 15 mL of deuterated hydrochloric acid and slowly warmed to room temperature. Add saturated brine and ethyl acetate, extract 2-3 times, combine organic phases, dry, filter, and concentrate under reduced pressure to obtain compound (IIa) (9.0g), yield 90%, MS detection, isotope abundance 98.8% .
Embodiment 2
[0044] Embodiment 2: the synthesis of 3,4-dimethoxyphenylacetonitrile-D2 (IIa):
[0045] The starting material 3,4-dimethoxyphenylacetonitrile (Ia) (10.0 g) was dissolved in 60 mL of dry THF and 100 mL of D 2 O, add pre-dried K 2 CO 3 (31.0g, 4eq), the reaction mixture was stirred at room temperature for 48h. Dry tert-butyl methyl ether was added, separated, and the organic phase was dried, filtered, and concentrated under reduced pressure to obtain compound (IIa) (9.5 g), with a yield of 95%. The isotopic abundance was 99.8% as detected by MS.
Embodiment 3
[0046] Embodiment 3: the synthesis of 3,4-dibenzyloxyphenylacetonitrile-D2 (IIb):
[0047]
[0048] The starting material 3,4-dibenzyloxyphenylacetonitrile (Ib) (5.0 g) was dissolved in 40 mL of dry THF and 50 mL of D 2 O, add pre-dried K 2 CO 3 (8.4g, 4eq), the reaction mixture was stirred at room temperature for 48h. Dry tert-butyl methyl ether was added, separated, and the organic phase was dried, filtered, and concentrated under reduced pressure to obtain compound (IIb) (4.8g), with a yield of 96%. The isotope abundance was 99.8% as detected by MS.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com