High-performance catalyst for preparing diol by hydrating epoxyalkane, preparation method and application
A technology of alkylene oxide water and catalyst, which is applied in the field of high-performance catalyst, preparation and application of alkylene oxide hydration to diol, can solve the problems of high water ratio and poor stability, and solve the problems of high water ratio and poor stability , excellent recycling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
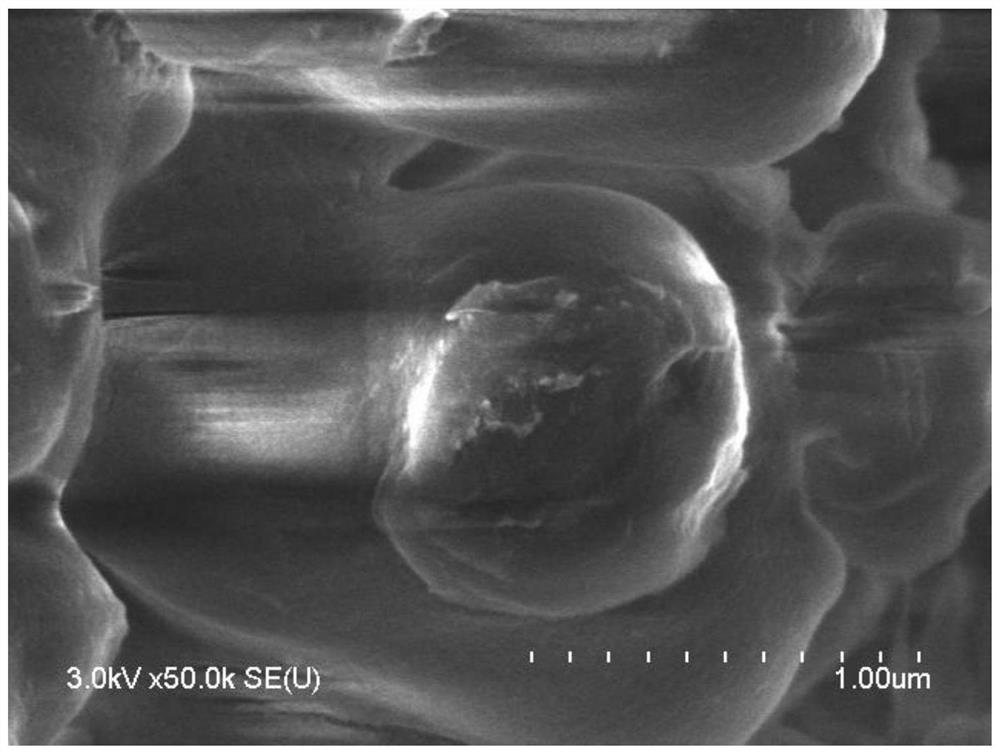
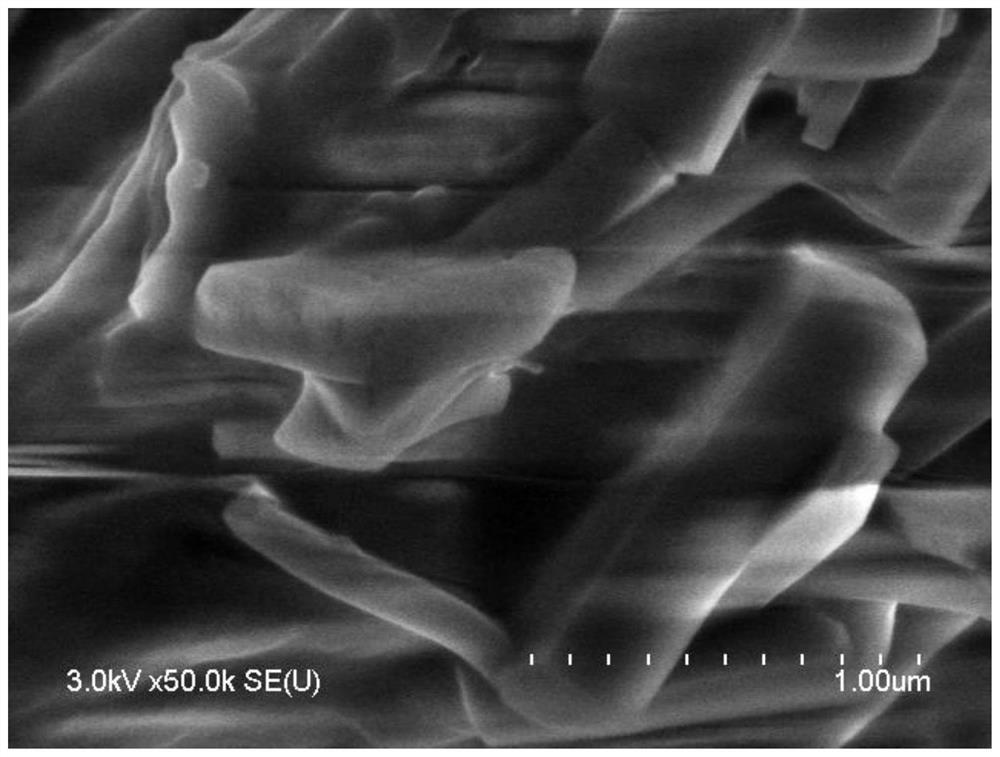
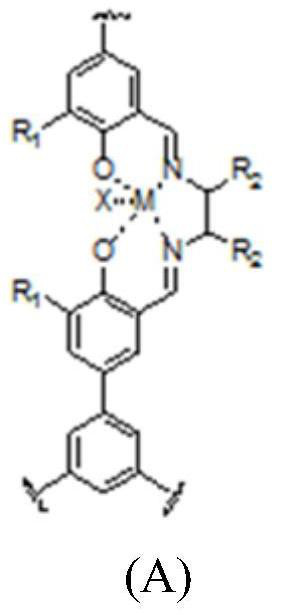
Image
Examples
Embodiment 1
[0031] Weigh 6 mmol of 1,3,5-tris(3-formyl-4-hydroxyphenyl)benzene and dissolve in CH 2 Cl2 Add a methanol solution containing 9 mmol ethylenediamine dropwise to it, reflux at 60°C for 1 hour and then separate, fully wash with methanol and dry; the obtained polymer is then dispersed in CH 2 Cl 2 Medium, N 2 Under atmospheric stirring, add 9mmol Co(OAc) 2 Methanol solution, separated after 10h of reaction, CH 2 Cl 2 Wash thoroughly with methanol solution and dry; then disperse the obtained product in CH 2 Cl 2 In, under stirring, drop into CH containing 9 mmol hexafluoroferrocene phosphate 2 Cl 2 The solution was separated after being stirred openly for 10 hours, washed thoroughly, and dried to obtain catalyst A.
Embodiment 2
[0033] Weigh 6 mmol of 1,3,5-tris(3-formyl-4-hydroxy-5-tert-butylphenyl)benzene and dissolve in CH 2 Cl 2 Add an ethanol solution containing 9 mmol cyclohexanediamine dropwise to it, reflux at 80°C for 1 h, then separate, wash with ethanol and dry; the obtained polymer is then dispersed in CH 2 Cl 2 In, add containing 9mmol Rh(Cl) 3 water / ethanol solution, separated after 10h of reaction, CH 2 Cl 2 and water / ethanol and dried thoroughly; the resulting product was then dispersed in CH 2 Cl 2 , drop into CH containing 9 mmol silver tetrafluoroborate under stirring 2 Cl 2 The solution was separated after reacting for 10 h, fully washed and dried to obtain catalyst B.
Embodiment 3
[0035] Weigh 2mmol of 1,3,5-tris(3-formyl-4-hydroxyphenyl)benzene and 4mmol of 1,3,5-tris(3-formyl-4-hydroxyl-5-tert-butylphenyl) ) Benzene dissolved in CH 2 Cl 2 Add dropwise an ethanol solution containing 2mmol of ethylenediamine, 3mmol of cyclohexanediamine and 4mmol of phenylenediamine to it, reflux at 80°C for 1h and then separate, fully wash with ethanol and dry; the obtained polymer is redispersed in CH 2 Cl 2 Medium, N 2 Under the atmosphere stirring, add containing 7mmol Co(OAc) 2 and 2mmol Rh(Cl) 3 Methanol solution, separated after 10h of reaction, CH 2 Cl 2 and methanol and dried thoroughly; the resulting product was then dispersed in CH 2 Cl 2 In, under stirring, CH containing 5mmol silver hexafluorophosphate and 4mmol silver tetrafluoroborate was added dropwise 2 Cl 2 solution, separated after 10 h of reaction, fully washed, and dried to obtain catalyst C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com