Zwitterionic hydrogel capable of recruiting type II collagen and its preparation method and application
A zwitterion, hydrogel technology, applied in the direction of prosthesis, peptide, medical science, etc., can solve the problem of not being able to recruit type II collagen, and achieve the effect of easy mass production and application promotion, improved repair effect, and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In this embodiment, the preparation method of active ester functional monomer is provided, and the steps are as follows:
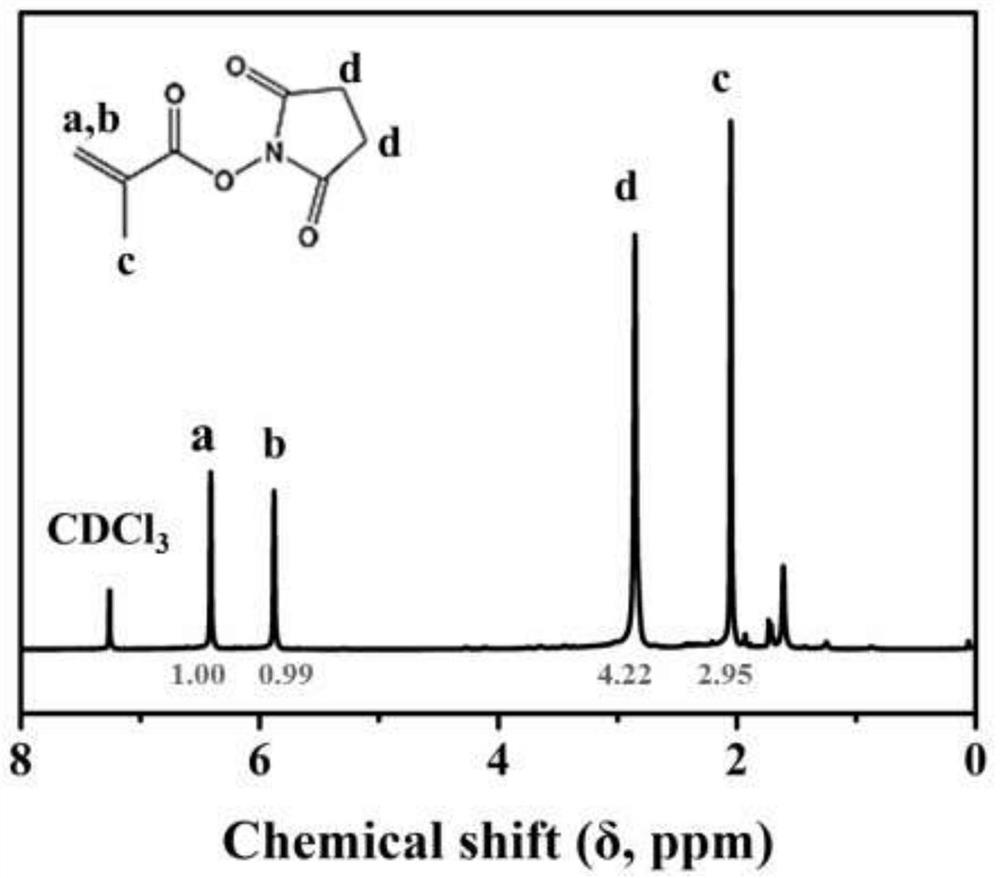
[0038] Dissolve N-hydroxysuccinimide (NHS, 1.1736g, 12mmol) and triethylamine (1,650μL, 12mmol) in 5mL of chromatographic grade dichloromethane, and stir for 30 minutes under ice-bath conditions. At the same time, methyl Acryloyl chloride (1 mL, 11 mmol) was dissolved in 5 mL of chromatographic grade dichloromethane and added dropwise to the previously prepared solution at a rate of 1 drop per 10 seconds. After the solution was added dropwise, the reaction system was gradually returned to room temperature and the reaction was continued for 12 h. After the reaction, wash with distilled water 3 times, 5 mL each time; then dry with anhydrous sodium sulfate, concentrate the obtained organic solution by rotary evaporation, and dissolve in ethyl acetate / n-hexane (1 / 3) mixed solution Precipitation gave a white product. Its reaction equation is shown in f...
Embodiment 2
[0042] In this embodiment, a method for preparing a zwitterionic hydrogel capable of recruiting type II collagen is provided, and the steps are as follows:
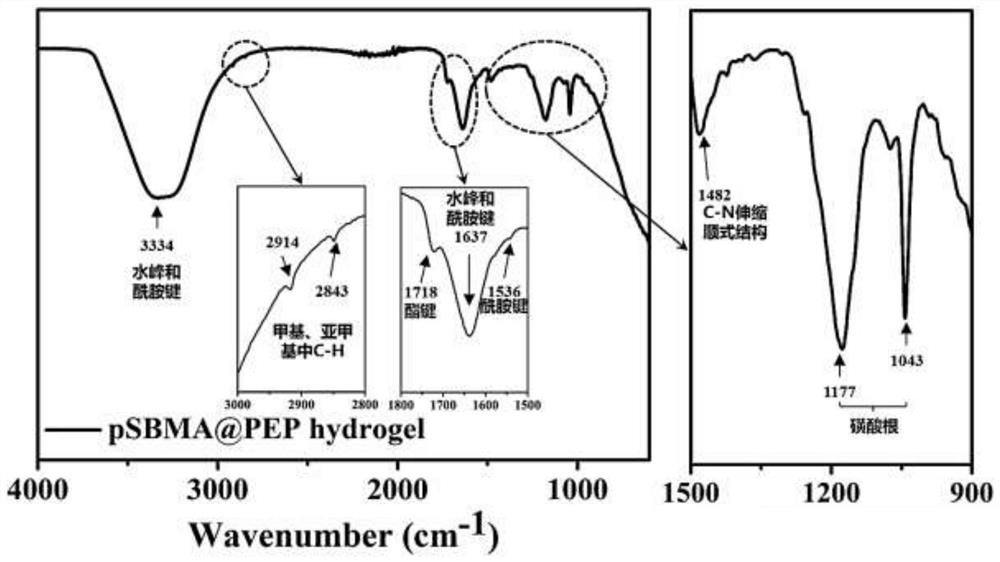
[0043] (1) Preparation of functionalized sulfobetaine hydrogel
[0044] Dissolve sulfobetaine monomer, active ester monomer, cross-linking agent MBA and photoinitiator I2959 in 15 mL of ultrapure water, and ultrasonically dissolve all components fully. Add the solution to a 24-well plate with 1 mL of liquid per well. Finally, it is cross-linked into a gel under ultraviolet light. Wherein, the chemical structure of the zwitterionic monomer used as the main structure of the hydrogel is shown in formula (II):
[0045]
[0046] (2) Preparation of type II collagen-binding peptide modified sulfobetaine hydrogel
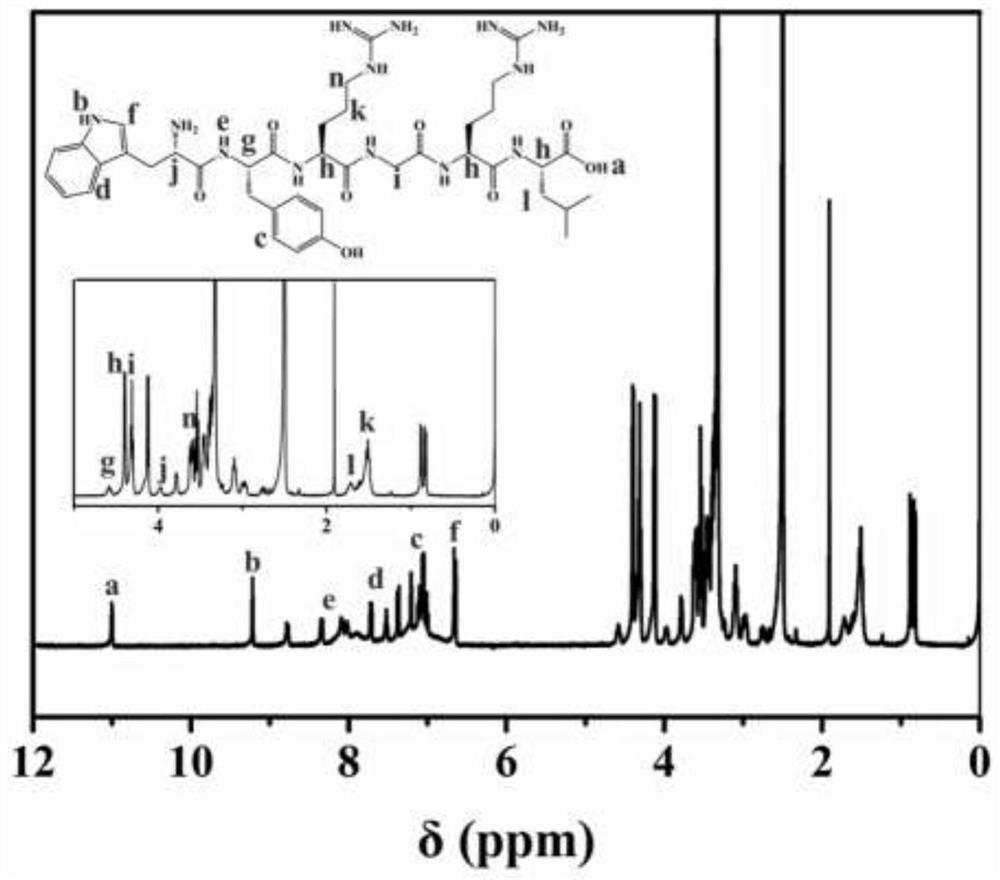
[0047] Soak the functionalized sulfobetaine hydrogel prepared in step 1 in PBS buffer, and change the water every 6 hours until the unreacted monomers are completely removed. Meanwhile, the WYRGRL polypeptide wa...
Embodiment 3
[0050]In this example, the synthesis scheme of the active ester monomer and the preparation of the hydrogel are basically the same as in Example 1 and Example 2, the difference is that the sulfobetaine monomer in Example 2 is replaced by phosphorylcholine Monomer, to prepare a zwitterionic hydrogel with the ability to recruit type II collagen, denoted as pMPC@PEP. Wherein, the chemical structure of the zwitterionic monomer used as the main structure of the hydrogel is shown in formula (III):
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com