A kind of metformin salt and its preparation method and application
A metformin salt and a metformin technology are applied in the field of metformin salt and its preparation, can solve the problems of unfavorable salt type sales, complex structure, high price and the like, and achieve the effects of simple and environmentally friendly preparation method, good solubility and easy preservation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: Preparation of metformin and 1-naphthalene acetate
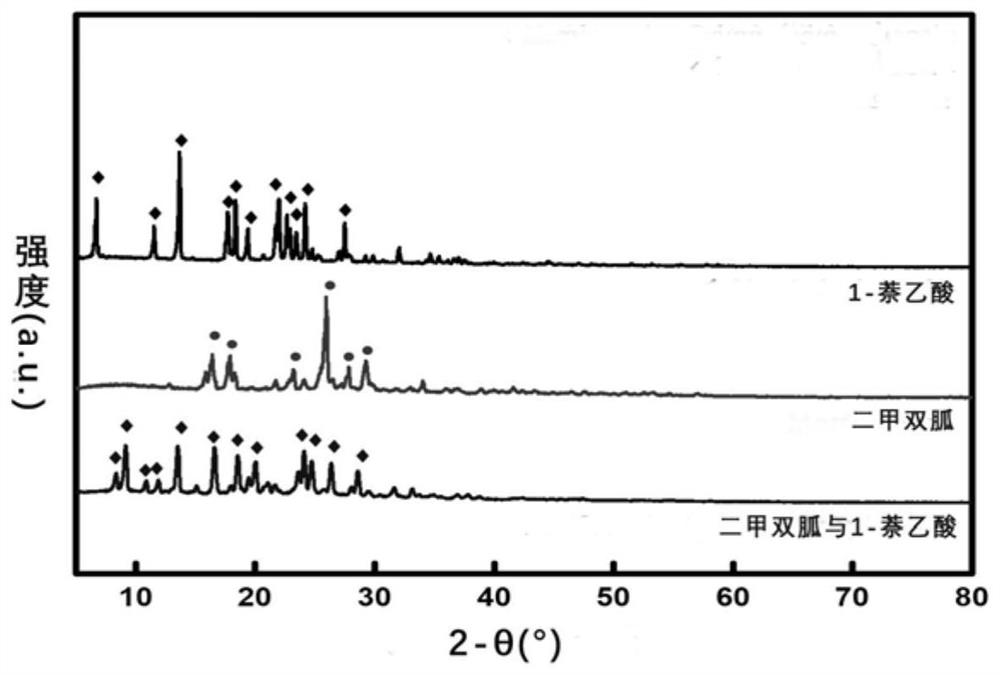
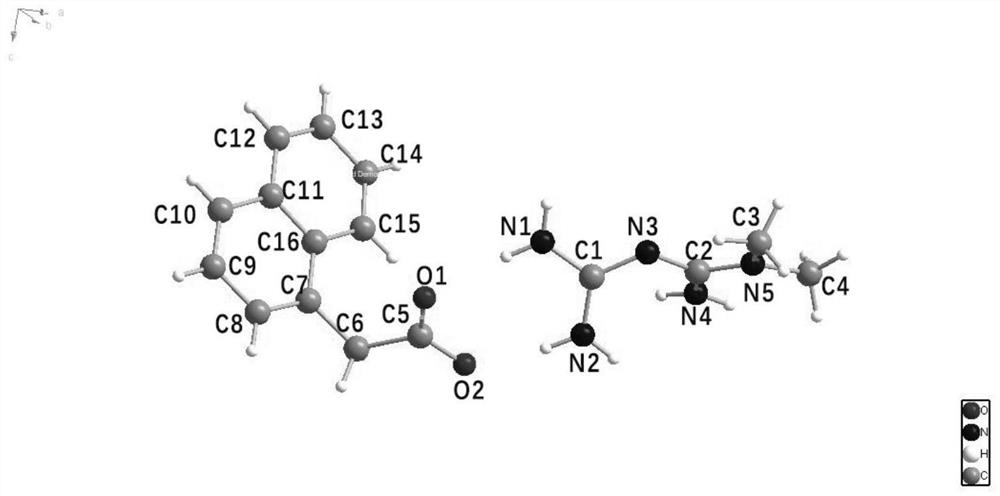
[0069] Take 0.0430g of commercially available metformin free base, pharmaceutical excipients and 0.0621g of 1-naphthalene acetic acid, drop 1-2 drops of absolute ethanol and mix them evenly, put them in a 100mL ball mill, and add 2 zirconia balls with a diameter of 22mm. , cover the sealing cover, put it into the ball mill, set the instrument parameters to the speed of 35s -1 , run for 5 minutes, pass through a 154 μm stainless steel mesh, and obtain 0.58 g of the product metformin naphthalene acetate powder, with a yield of 98%.
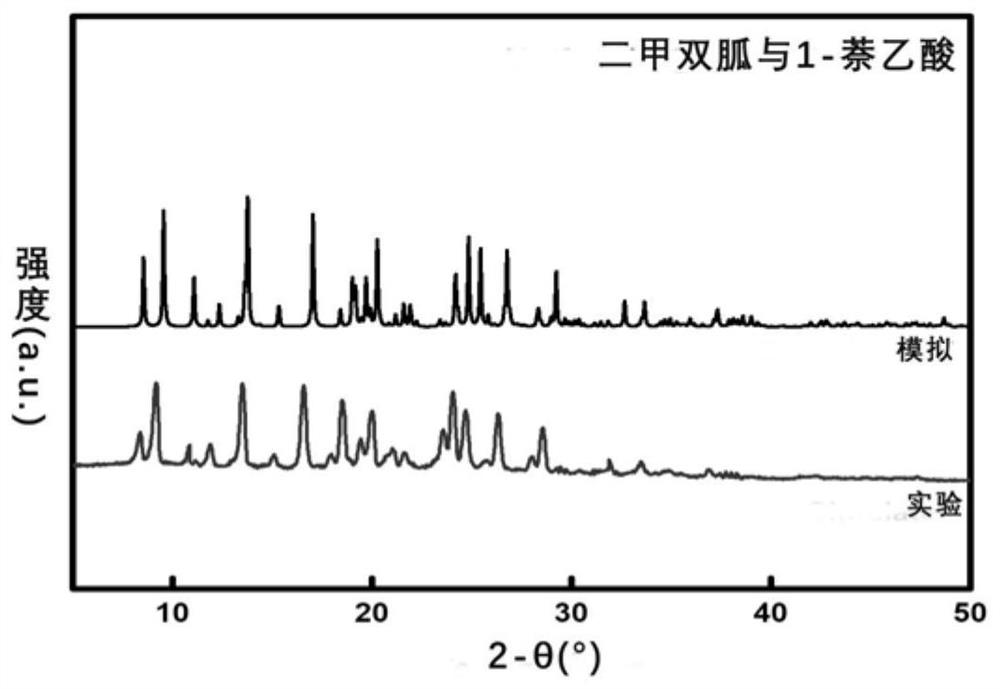
[0070] The product metformin obtained in Example 1 and the yellow powder of 1-naphthalene acetate, the raw material 1-naphthaleneacetic acid, and the free base of metformin are respectively subjected to powder X-ray diffraction analysis, and the results are as follows: figure 1 shown. from figure 1 It can be seen that the characteristic diffraction peaks of the obtained yellow...
Embodiment 2
[0074] Example 2: Preparation of metformin and 2-naphthoate
[0075] Take 0.0430g of metformin free base, pharmaceutical excipients and 0.0574g of 2-naphthoic acid, drop 1-2 drops of absolute ethanol and mix them evenly. After putting the sealing cover on, put it into a ball mill, set the instrument parameters to rotate speed 35s-1, run for 5min, pass through a 154 μm stainless steel screen, and obtain the powder of metformin and 2-naphthoate.
[0076] The powders obtained from metformin and 2-naphthoic acid salt, 2-naphthoic acid and metformin free base were analyzed by powder X-ray diffraction respectively, and the results were as follows Image 6 As shown in the figure, it can be seen that the characteristic diffraction peaks of metformin and 2-naphthoate, metformin free base and 1-naphthoacetic acid are different, which means that the prepared metformin and 2-naphthoate are a new kind of the solid form.
[0077] The prepared metformin and 2-naphthoate were fully dissolve...
Embodiment 3
[0080] Example 3: Preparation of metformin and 1-hydroxy-2-naphthoate
[0081] Take 0.0430g of metformin free base, pharmaceutical excipients and 0.0627g of 1-hydroxy-2-naphthoic acid, drop 1-2 drops of absolute ethanol and mix them uniformly, put them in a 100mL ball mill, add 2 22mm diameter dioxide Zirconium ball grinding beads, cover the sealing cover, put it into the ball mill, set the instrument parameters to the speed of 35s -1 , run for 5 min, pass through a 154 μm stainless steel mesh to obtain the powder of the product metformin salt.
[0082] The powders prepared by metformin and 1-hydroxy-2-naphthoate, 1-hydroxy-2-naphthoic acid and metformin free base were analyzed by powder X-ray diffraction respectively, and the results were as follows Figure 11 As can be seen from the figure, the characteristic diffraction peaks of metformin and 1-hydroxy-2-naphthoic acid salt and metformin free base and 1-hydroxy-2-naphthoic acid are all different, indicating that the prepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com