Application of ursolic acid derivatives in the preparation of medicines for treating nervous system diseases
A technology of ursolic acid and derivatives, applied in the field of medicine, can solve the problems of difficulty in obtaining derivatives, lack of active groups in the parent nucleus, incomplete chemical and pharmacological research on ursolic acid derivatives, etc., and achieve good repair of nerve cell damage. and anti-neuroinflammatory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
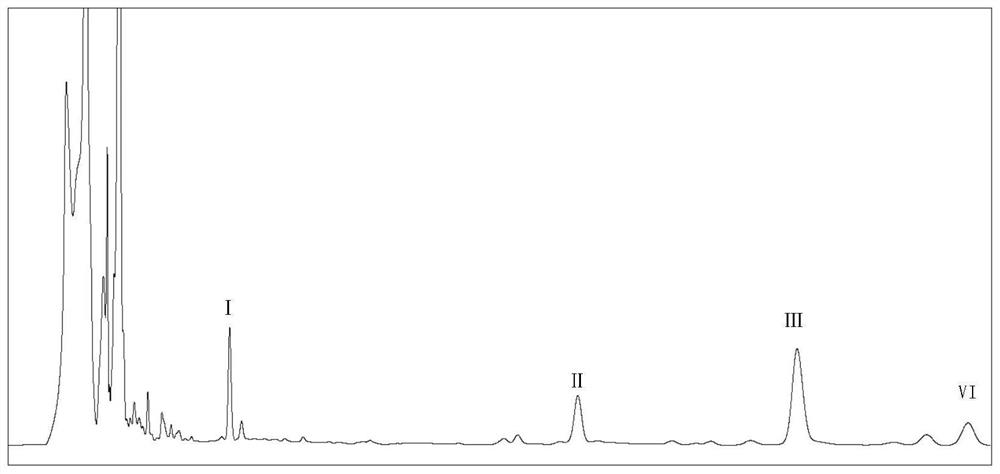
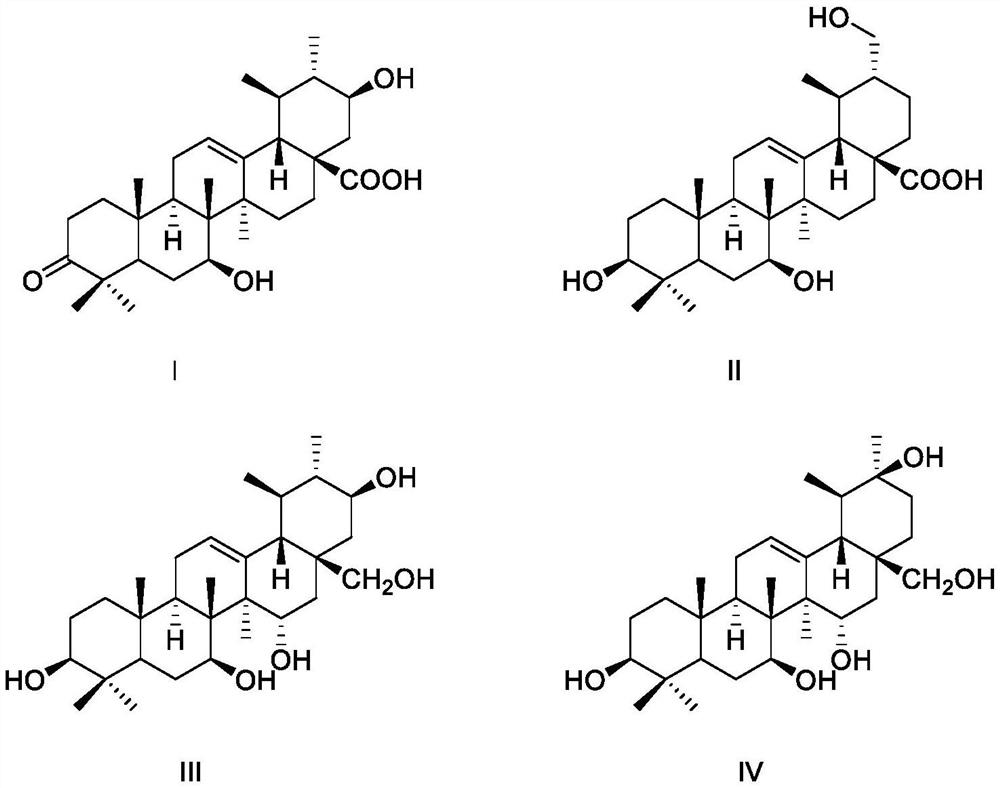
[0020] Embodiment 1, structural formula is the preparation of the compound of formula I-formula IV
[0021] The invention adopts a microbial conversion method, uses ursolic acid as a raw material, and undergoes steps such as fermentation, extraction, and separation to prepare the compound of the invention. Circinella genus strains can be purchased from the Chinese Academy of Sciences Microbial Culture Collection Center (CGMCC), using potato medium, and stored on a solid slant medium in a refrigerator at 4°C.
[0022] Taking Circinella muscae CGMCC 3.2695 as an example, the process of preparing the compound whose structural formula is formula I-Formula IV is as follows:
[0023] 1) Fermentation, transformation and extraction
[0024] Put Circinella muscae CGMCC 3.2695 into two 250mL Erlenmeyer flasks (containing 100mL potato culture medium) as seed solution. After shaking and culturing on a shaker at 160 rpm and 26°C for 1 day, when the mycelium growth is in a vigorous stage,...
Embodiment 2
[0031] Example 2 Compounds Ⅰ-Ⅳ have repairing and protective activity on nerve cells damaged by hydrogen peroxide
[0032] (1) Experimental materials
[0033] CO 2 Incubator (Jouan IGO150); Microplate reader (Bio-TEK ELx800); Fluorescent inverted microscope (OlympusIX51); MTT cell proliferation and cytotoxicity detection kit (Biotech Institute), DMEM high glucose medium (Gibcol BRL ), fetal bovine serum, dimethyl sulfoxide (DMSO), trypsin (Shanghai Bioengineering Co., Ltd.), 30% hydrogen peroxide (H 2 o 2 ) (Tianjin Ruijinte Chemicals Co., Ltd.), SH-SY5Y cells (Tumor Institute, Chinese Academy of Medical Sciences).
[0034] (2) Experimental method
[0035] The MTT method was used to determine the H of each test compound 2 o 2 Influence of damaged SH-SY5Y cell viability: count the cells after digestion with trypsin, adjust the cell density of the cell suspension to 5×10 4 cells / mL, add 200 μL to each well of a 96-well culture plate, place in 5% CO 2 , 37°C constant temp...
Embodiment 3
[0042] Example 3 Anti-neuroinflammatory activity of compounds Ⅰ-Ⅳ of the present invention
[0043] 1) Experimental materials
[0044]Instruments and reagents: CO2 incubator (Jouan IGO150); microplate reader (Bio-TEK ELx800); fluorescent inverted microscope (Olympus IX51); MTT cell proliferation and cytotoxicity detection kit (Beiyuntian Biotechnology Institute), RPMI 1640 Medium (Gibcol BRL), microglial cells BV-2, RNase A, fetal bovine serum, dimethyl sulfoxide (DMSO), trypsin (Shanghai Bioengineering Co., Ltd.).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com