Monoclonal antibody of filamentous phage pVIII protein and application thereof
A filamentous bacteriophage and monoclonal antibody technology, applied in the field of biomedicine, can solve the problem of no monoclonal antibody, and achieve the effects of excellent immunogenicity, single biological activity, high specificity and high titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Synthesis and Identification of Immunogen
[0053] 1. Preparation of immunogen
[0054] (1) The antigenic epitopes for the pVIII protein are all located on the outer surface of the phage capsid (the polypeptide sequences are all at the N-terminus, and the sequence is shown in SEQ ID NO.5: AEGDDPAKAAFDSLQASAT) to design and synthesize the outer membrane of the filamentous phage pVIII capsid protein The polypeptide part Cys-Ahx-AEGDDPAKAAFDSLQASAT, the polypeptide is named TC21;
[0055] (2) Coupling the above synthesized polypeptide with KLH and BSA carrier proteins respectively to obtain the immunogen.
[0056] 2. Immunogen identification
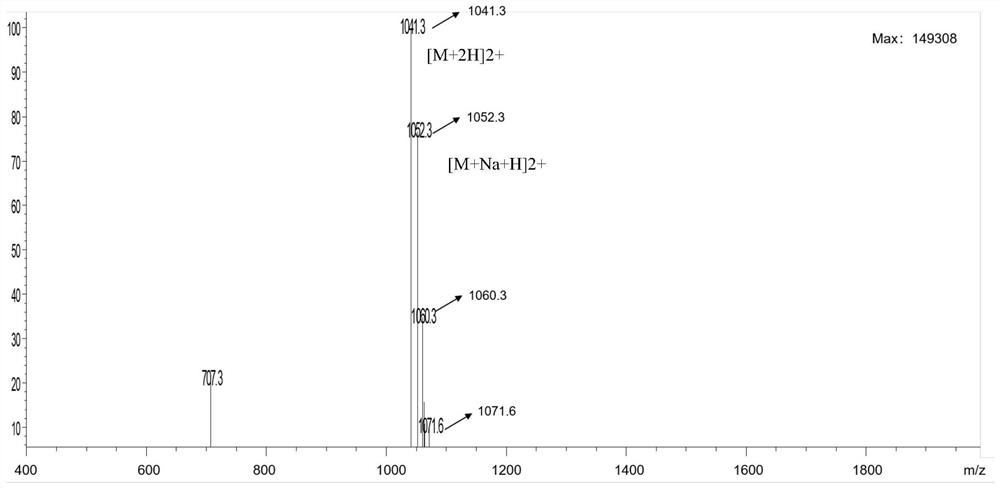
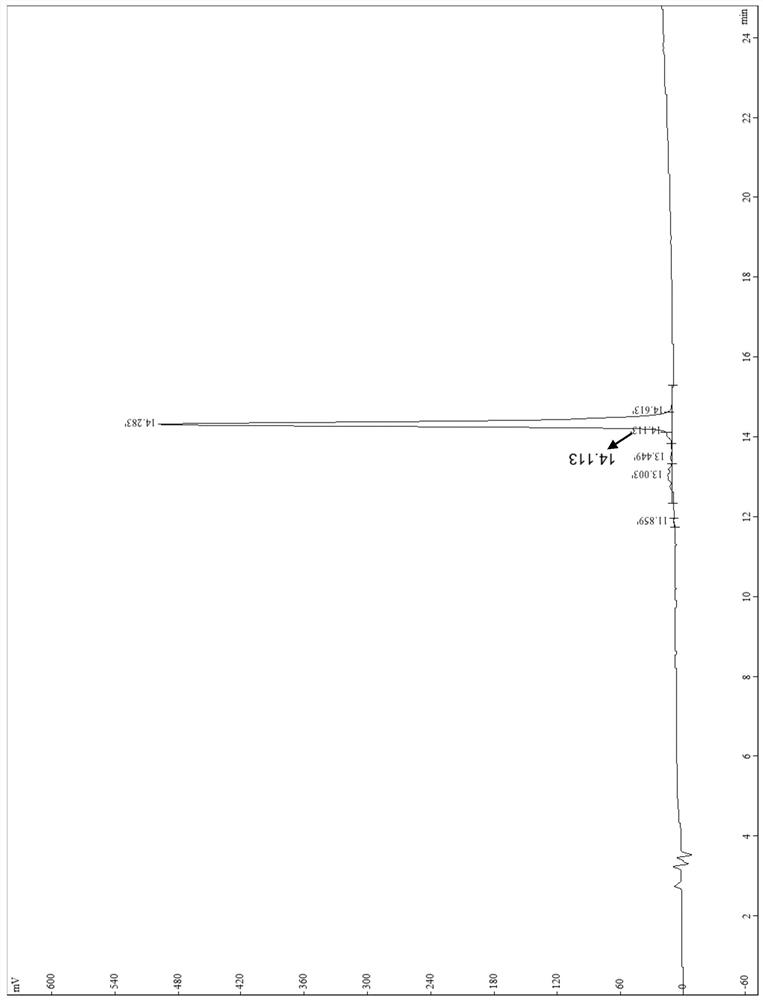
[0057] The polypeptide synthesized in step 1 was identified by mass spectrometry and high performance liquid chromatography, and the identification results were as follows: figure 1 (mass spectrometry) and figure 2 (High Performance Liquid Chromatography), the results showed that the polypeptide was synthesized success...
Embodiment 2
[0059] Example 2 Preparation of Monoclonal Antibody Against Filamentous Phage pVIII Protein
[0060] (1) Immunization of animals
[0061] Animals: Balb / c mice, screening conditions: female, body weight 18-20g, age 6-8 weeks.
[0062] The conjugates of the polypeptide TC21 prepared in Example 1 and the carrier protein (polypeptide group) and the M13KO7 helper phage (phage group) were used as immunogens to immunize animals, and five groups were set up, and 10 BALB / c mice were immunized in each group. Among them, the immunization of the M13KO7 helper phage group was divided into three groups with different dose concentrations (the first group, the second group, and the third group); The immunization was divided into two groups (BSA-CT21 as the fourth group, and KLH-CT21 as the fifth group). The mice were fed for one week before immunization, blood was collected from the orbital venous plexus, centrifuged, and the serum was taken as a negative control.
[0063] The immunization...
Embodiment 3
[0135] Example 3 Subtype Identification and Sequencing of Monoclonal Antibodies
[0136] The subtype identification of the monoclonal antibodies M5G8 and P8E4 prepared in Example 2 was carried out, and the specific method was as follows:
[0137] The subtype classification of the antibody was identified using the Pierce Rapid ELISAMouse mAb Isotyping Kit (product of Sigma, catalog number 19285). The subtype of the monoclonal antibody was detected, and the results of the immunoglobulin subtype of the monoclonal antibody are shown in Table 2.
[0138] Table 2 Antibody subtype identification
[0139]
[0140] The monoclonal antibodies M5G8 and P8E4 were sequenced, wherein the heavy chain variable region sequence of the monoclonal antibody M5G8 was shown in SEQ ID NO.1, and the light chain variable region sequence was shown in SEQ ID NO.3; the monoclonal The heavy chain variable region sequence of antibody P8E4 is shown in SEQ ID NO.2, and the light chain variable region sequen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com