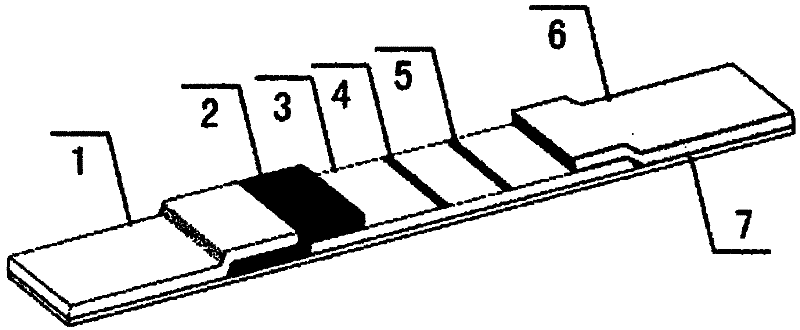
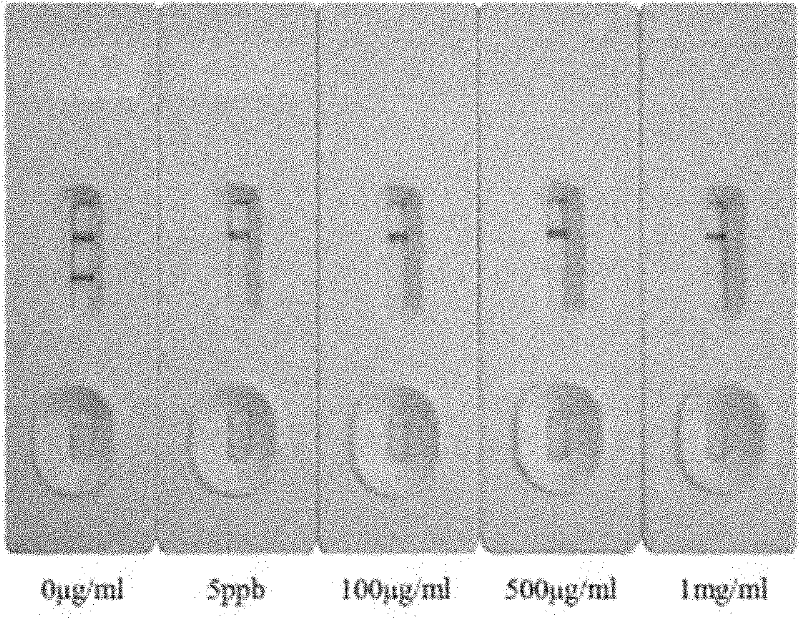
Sildenafil monoclonal antibody and colloidal gold chromatography test strip used for detecting sildenafil
A monoclonal antibody, sildenafil technology, applied in the direction of microorganism-based methods, analytical materials, measuring devices, etc., can solve the problems of no sildenafil monoclonal antibody report, long reaction time, high cost of experiments, etc. Achieve the effect of being convenient for human handling and quality control, easy to operate, and single biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A synthesizes N-acetate sildenafil (II-i)
[0051] (1) Alkylation: Add 20ml of dry dimethylformamide (DMF) and 1.708g, 3.6×10 -3 mol of sildenafil (IV-i), and through N 2 protection, stirred at room temperature, and after dimethylformamide (DMF) was completely dissolved, quickly weighed 0.173g, 4.32×10 -3 mol, concentration is 60% sodium hydride (NaH), and it is added in the described reaction bottle, stirs 60 minutes, until the solution produces without bubble, draws 1.202g, 7.2 * 10 with 1ml syringe then -3 mol of ethyl bromoacetate was slowly injected into the reaction flask, and after the injection was completed, it was stirred at room temperature for 12 hours, and then the reaction solution was poured into 60ml of water to disperse, extracted with 20ml×3 chloroform, and the extracts were combined to obtain Anhydrous Na 2 SO 4 Dry, then filter, and then concentrate under reduced pressure to obtain a solid, add 6ml of anhydrous ether to wash the solid, then filte...
Embodiment 2
[0065] The establishment of embodiment 2 hybridoma cell lines
[0066] Step 1 Animal Immunization
[0067] Artificially synthesized antigen N-sildenafil acetate-BSA was emulsified with an equal amount of complete Freund's adjuvant, and 0.2ml (1mg / ml) was subcutaneously injected into mice at multiple points. After 3 weeks, N-sildenafil acetate-BSA plus the same amount of Freund's incomplete adjuvant was fully mixed, and then subcutaneously injected 0.2ml (1mg / ml) into mice at multiple points. After 10 days, the tail was docked to collect blood One drop was used to measure the antibody titer, and mice with high titers were selected for fusion test. One month later, 0.1 ml of non-adjuvant antigen N-sildenafil acetate-BSA can be administered intravenously, and after 3 days, the mice are killed and spleens are taken for fusion.
[0068] A mouse myeloma line was induced from the Balb / c mouse line. Balb / c mice must be of pure line, both male and female, preferably 8-12 weeks old. ...
Embodiment 3
[0106] Example 3 In vivo induced ascites method to prepare monoclonal antibody
[0107] Usually, the method of producing monoclonal antibody in animals is adopted. Since the vast majority of animal hybridomas are obtained from the fusion of myeloma cells of Balb / c mice and spleen cells of the same strain, the animal used is of course the first choice of Balb / c mice. c mice. In this method, the hybridoma cells are inoculated into the peritoneal cavity of the mouse, the hybridoma is grown in the peritoneal cavity of the mouse, and ascites is produced, so a large amount of monoclonal antibody in the ascitic fluid can be obtained with a high antibody concentration. It can be seen that this method is easy to operate and economical, but ascites is often mixed with various miscellaneous proteins of mice, so in many cases it must be purified before use, and there is also the risk of contamination of animal viruses, so it is best to use SPF mice . The operation method is as follows: ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com