Detection method of propofol fumarate tenofovir isomer
A technology of propofol fumarate and tenofovir, which is applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of single detection structure and inability to meet the requirements of baseline separation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Establishment and condition optimization of detection methods:
[0085] Limit specification:
[0086] Isomeric impurities, according to general impurities, according to human drug registration technology International Coordination Conference (ICH) guidelines, limited to 0.10%, as a reference foundation for detection method development.
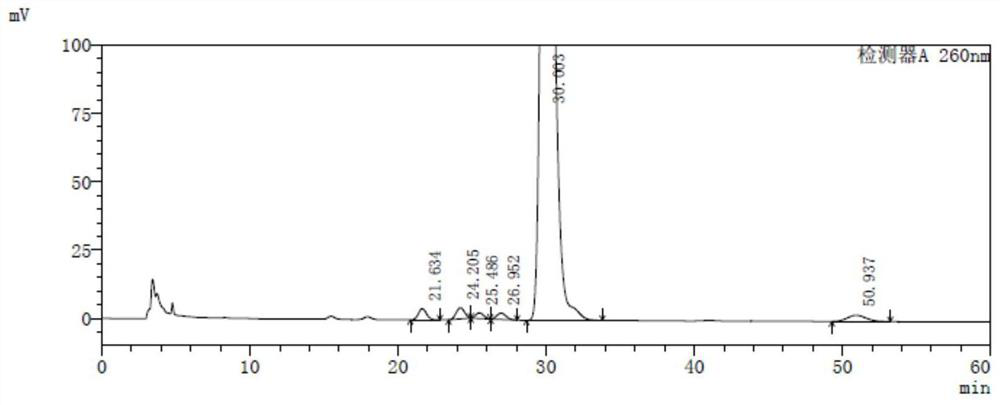
[0087] Each isomer impurity has a maximum absorption wavelength at around 260 nm, and the detection wavelength can be selected from 258 nm to 262 nm. The present embodiment is preferably 260 nm; when the amount of intake is 20 μl, each isomer impurity peak is a minimum of 0.5mV, It has been able to meet the sensitivity requirements of the method, so it is determined that the concentration of the tester is 0.5 mg / ml, the isomer gas concentration is 0.5 μg / ml, and each isomer impurity is formulated according to the concentration of the test. And impurities positioning solutions.
[0088] Screening of columns:
[0089] The concentration of...
Embodiment 2
[0112] Verify system applicability and exclusive
[0113] Solution preparation:
[0114] Blank solvent: absolute ethanol, diluent;
[0115] Isomeric stocks: 10 mg of TAF-6-A, TAF-6-A1 and TAF-6-A3, respectively, and are precisely determined, respectively, respectively, respectively, 20 ml of four different 20 ml, respectively. In the volumetric flask, the dilute agent is dissolved and diluted to the scale to obtain isomer reserve, wherein TAF-6-A, TAF (free state), TAF-6-A1 peak 1, TAF-6-A1 peak 2, TAF- 6-A3 peaks 1, TAF-6-A3 peak 2 and TAF-6-A2 peak concentrations are about 0.5 mg / ml, respectively;
[0116] Systematic Solution: Add 5 mg of the propylene glycassolidinolifuli raw material medicine to 20 mg, precisely 20mL volumetric flask, add 0.2ml of the isomer reserve, dissolve and dilute to the scale, Get systematic applicability solution, where fumarate propanol replacement Novir concentration is about 0.5 mg / ml, TAF-6-A, TAF-6-A1 peak 1, TAF-6-A1 peak 2, TAF-6- A3 peaks 1...
Embodiment 3
[0127] Verify detection limit and quantitative limit:
[0128] Solution preparation:
[0129] Blank solvent: absolute ethanol, diluent;
[0130] Isomeric stocks: 10 mg of TAF-6-A, TAF-6-A1 and TAF-6-A3, respectively, and are precisely determined, respectively, respectively, respectively, 20 ml of four different 20 ml, respectively. In the volumetric flask, the dilute agent is dissolved and diluted to the scale to obtain isomer reserve, wherein TAF-6-A, TAF (free state), TAF-6-A1 peak 1, TAF-6-A1 peak 2, TAF- 6-A3 peaks 1, TAF-6-A3 peak 2 and TAF-6-A2 peak concentrations are about 0.5 mg / ml, respectively;
[0131] Isomeric positioning solution: 1 ml of each of the numeral reserve is used to separate four different 50 mL of volumetric flask, diluted to the scale, shake well with a diluent, to obtain an isomer-locating solution, wherein TAF-6-A, Taf (free state), TAF-6-A1 peak 1, TAF-6-A1 peak 2, TAF-6-A3 peak 1, TAF-6-A3 peak 2 and TAF-6-A2 peak concentrations are about 10 μg / ml...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com