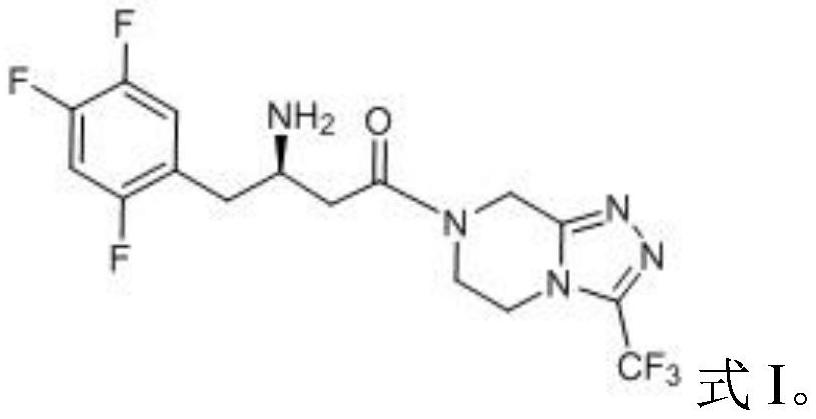
Recycling method of acetyl Meldrum's acid derivative degradation waste
A technology of Acetyl McBurney's Acid and its derivatives, which is applied in the field of resource recycling, and can solve problems such as environmental pollution, the inability to use sitagliptin API production, and the increase in the production cost of sitagliptin API
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
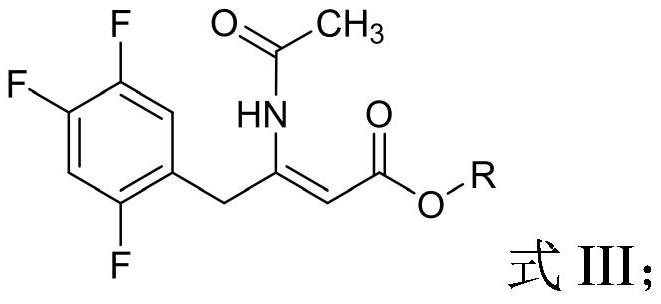
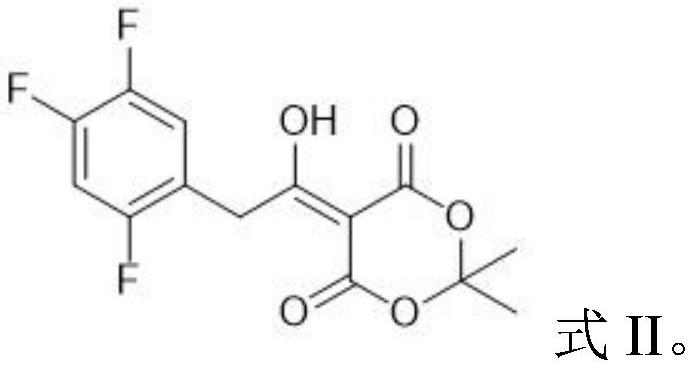
[0057] Take 8.00g of the oily waste produced by the degradation of Compound II and preheat at 60°C for 30min to obtain a preheated material; dissolve the preheated material in 80mL of methanol and heat to reflux under stirring (system temperature is 105~110 ℃), maintain reflux state and carry out alcoholysis reaction 60h (TLC monitors reaction process); Heated to 55-60°C under stirring conditions, and carried out ammonolysis reaction for 16 hours (monitoring the reaction process by TLC); after the reaction, the obtained system was cooled to room temperature, and the solvent was recovered by distillation under reduced pressure, and the residue was dissolved in 60 mL of ethyl acetate, The resulting mixture was washed 3 times with water (80mL×3), the organic layer was separated and the solvent was recovered by distillation under reduced pressure, the residue was dissolved in 20mL of toluene, and 8mL of acetic anhydride was added, and the resulting system was heated to reflux (th...
Embodiment 2
[0060] Take 12.00g of the oily waste produced by the degradation of Compound II and preheat it at 60°C for 30min to obtain the preheated material; dissolve the preheated material in 80mL of methanol and heat to reflux under stirring (the system temperature is 105~110 ℃), maintain reflux state and carry out alcoholysis reaction 72h (TLC monitors reaction process); Heated to 55-60°C under stirring conditions, and carried out ammonolysis reaction for 24 hours (monitoring the reaction process by TLC); after the reaction, the obtained system was cooled to room temperature, and the solvent was recovered by distillation under reduced pressure, and the residue was dissolved in 100 mL of ethyl acetate, The resulting mixture was washed 3 times with water (80mL×3), the organic layer was separated and the solvent was recovered by distillation under reduced pressure, the residue was dissolved in 30mL of toluene, and 10mL of acetic anhydride was added, and the resulting system was heated t...
Embodiment 3
[0063]Get 16.00g of the oily waste produced by the degradation of Compound II and preheat at 60°C for 30min to obtain a preheated material; dissolve the preheated material in 80mL of ethanol and heat to reflux under stirring (system temperature is 105~110 ℃), maintain reflux state and carry out alcoholysis reaction 60h (TLC monitors reaction process); Heated to 55-60°C under stirring conditions, and carried out ammonolysis reaction for 24 hours (monitoring the reaction process by TLC); after the reaction, the obtained system was cooled to room temperature, and the solvent was recovered by distillation under reduced pressure, and the residue was dissolved in 100 mL of ethyl acetate, The resulting mixture was washed 3 times with water (120mL×3), the organic layer was separated and the solvent was recovered by distillation under reduced pressure, the residue was dissolved in 30mL of toluene, and 10mL of acetic anhydride was added, and the resulting system was heated to reflux (t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com