Small molecule peptide as well as preparation method and application of small molecule peptide as nano drug carrier
A technology of nano-drug loading and small molecule peptides, which is applied in the direction of nano-drugs, medical preparations containing active ingredients, nanotechnology, etc., can solve the problems of difficult self-assembly preparation, application limitations, poor material biocompatibility, etc., and achieve cost-effective Low cost, good biocompatibility, and convenient purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
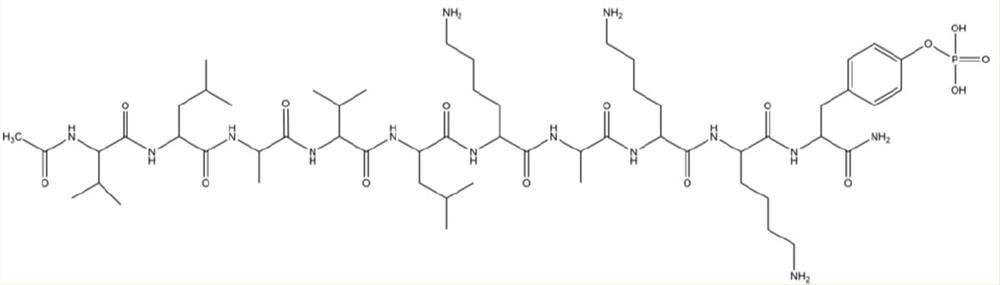
[0058] Example 1: Ac-LVVLKKK(pY)-NH 2 Preparation of peptides
[0059] Step 1: Distill N,N-dimethylformamide (DMF) and piperidine solvents
[0060] Distill the purchased DMF solution under reduced pressure at 60°C to obtain pure DMF solvent; add a small amount of CaH to the purchased piperidine 2 Heating to reflux for 1-2 hours, receiving the fraction at the boiling point (106°C) to obtain pure piperidine solvent.
[0061] Step 2: Preparation of amino acids, resins, activators, capping agents, and deprotecting agents
[0062] Calculated on the polypeptide solid-phase synthesizer to prepare 0.25mmol / L Ac-LVVLKKK(pY)-NH 2 Amounts of amino acids and other reagents required:
[0063] Leu (leucine): 0.78 g dissolved in 11 mL DMF;
[0064] Lys (lysine): 1.50 g dissolved in 16 mL DMF;
[0065] Tyr(PO 3 h 2 ) (phosphorylated tyrosine): 2.67 g dissolved in 28 mL DMF
[0066] Val (valine): 0.75 g dissolved in 11 mL DMF;
[0067] Resin (loaded at 0.6 mmol / g): 0.417 g;
[0068]...
Embodiment 2
[0075] Example 2: Ac-LVVLKKK(pY)-NH 2 Preparation of drug-loaded carrier
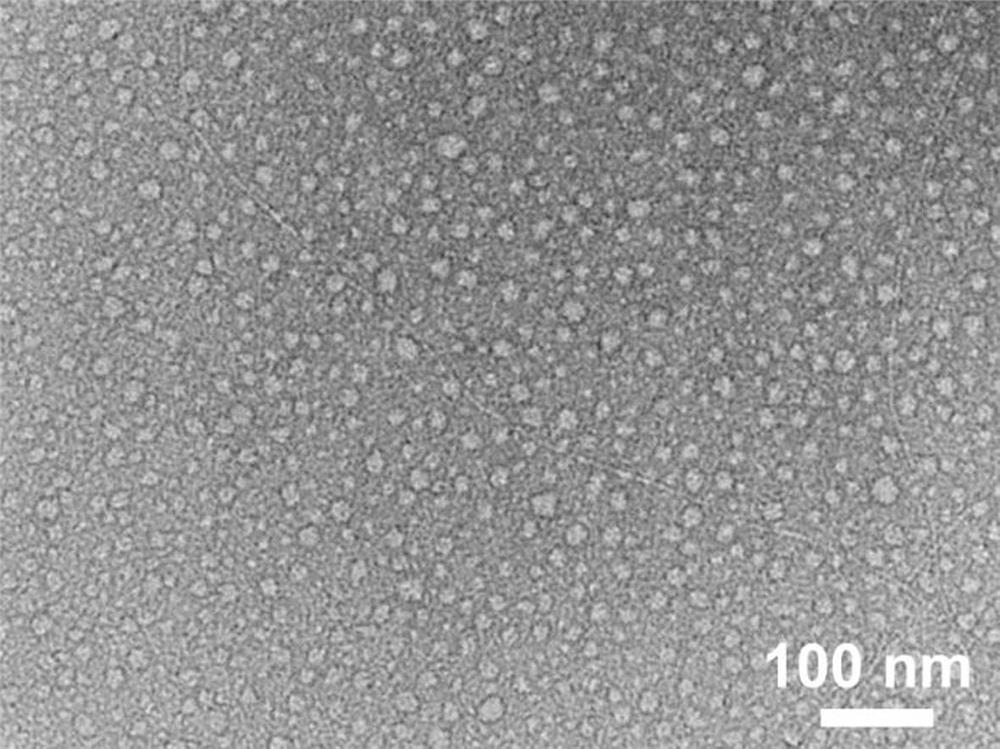
[0076] Weigh 0.59 mg of peptide Ac-LVVLKKK(pY)-NH 2 , adding 1 mL of Hepes buffer (pH7.4) to obtain a peptide solution with a concentration of 0.5 mmol / L, sonicating for 10 min, and standing at room temperature for 24 h, the observation results of transmission electron microscopy (TEM) showed that the peptides had self-assembled at this time A spherical nanoparticle structure is formed, while some nanofibers ( image 3 ).
Embodiment 3
[0077] Example 3: Ac-LVVLKKK(pY)-NH 2 Changes in Self-Assembled Morphology of Drug Carriers Encapsulating DOX
[0078] The specific detection method is as follows:
[0079] Detection of self-assembly morphology (TEM) of drug-loaded peptides in Hepes buffer after adding DOX:
[0080] Prepare Ac-LVVLKKK(pY)-NH with concentrations of 0.1 mmol / L, 0.5 mmol / L and 1 mmol / L 2 Add 1 mL of each peptide solution, and add 0.02 mL of DOX respectively, so that the final concentration of DOX is 0.12 mg / mL. Sonicate for 10 min, place at room temperature for 24 h, and observe with TEM. The results showed that Ac-LVVLKKK(pY)-NH 2 Peptide solution, after adding DOX, the nanofibrous structure disappears, and finally self-assembles into spherical nanoparticles loaded with drugs, such as Figure 4 , Figure 5 , Image 6 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com