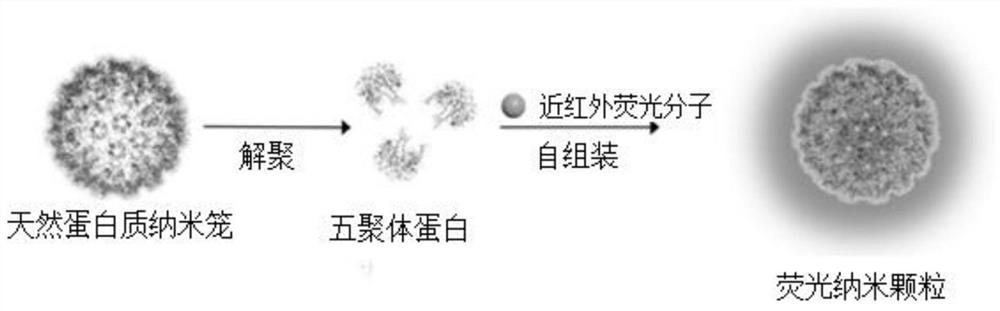
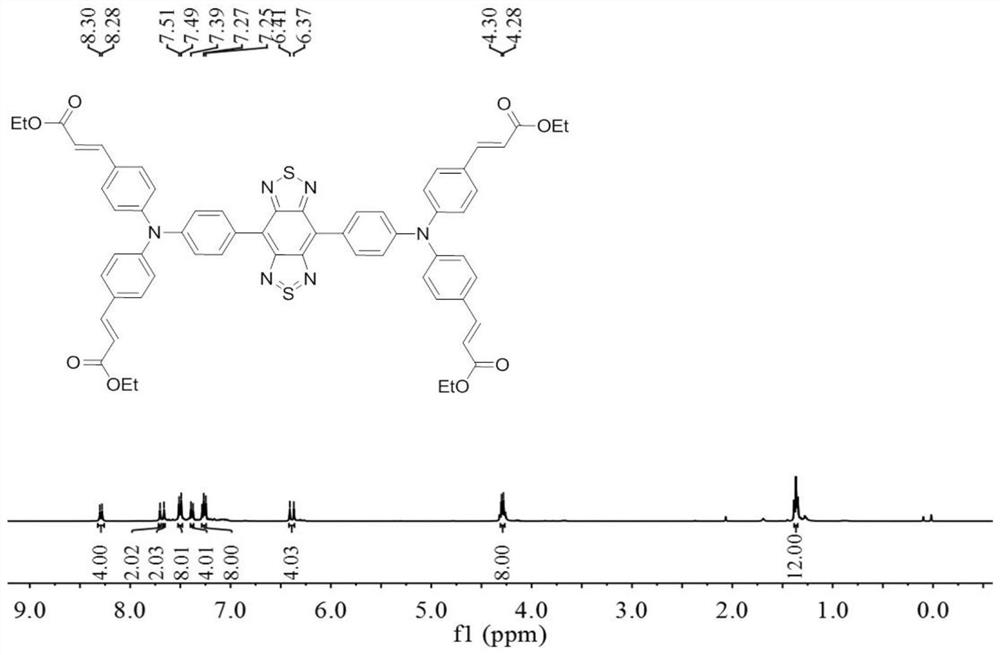
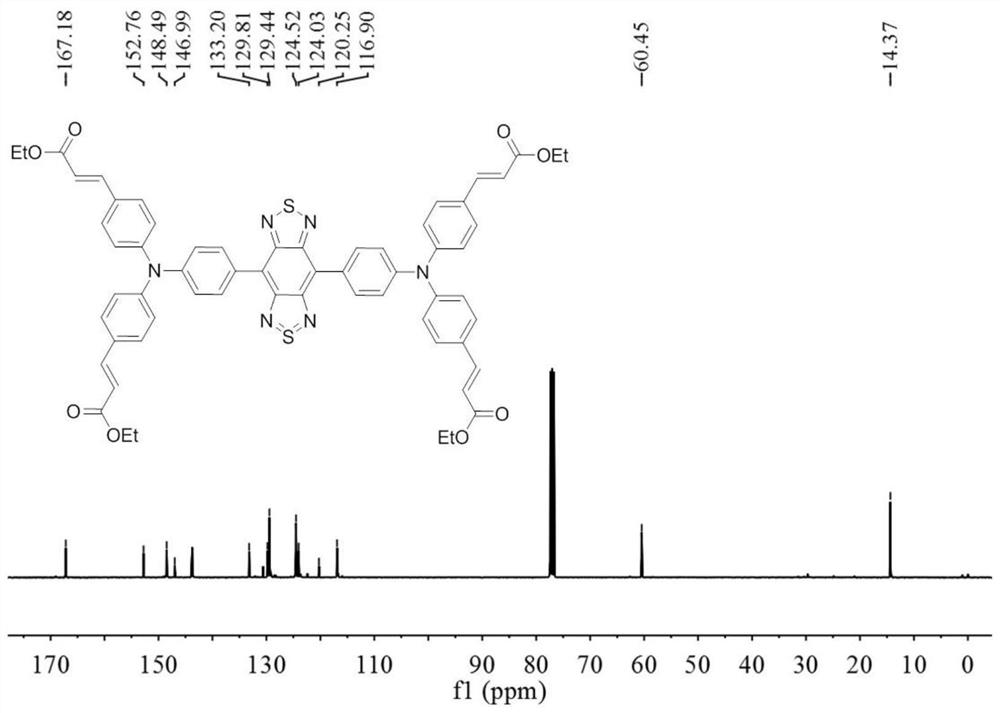
Near-infrared two-region fluorescent molecule containing benzobithiadiazole and preparation method thereof, fluorescent nano-particles and preparation method and application thereof
A benzobisthiadiazole and fluorescent nanoparticle technology, which is applied in the field of fluorescent materials, can solve the problems of low fluorescence stability, poor biocompatibility of organic fluorescent nanoparticles, and limitations in popularization and application, and achieves good fluorescence stability and convenience. Large-scale production, bright fluorescent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The invention provides a preparation method of the benzobisthiadiazole-containing near-infrared two-region fluorescent molecule, comprising the following steps:
[0049] (1) Under anhydrous and oxygen-free conditions, 4-bromotriphenylamine and phosphorus oxychloride were subjected to Vilsmeier-Haack reaction in N,N-dimethylformamide, and separated by chromatographic column to obtain formula III respectively compound and the compound shown in formula IV;
[0050]
[0051] (2) Under anhydrous and oxygen-free conditions, carry out a Witting reaction between the compound shown in formula III or the compound shown in formula IV and (triphenylphosphine) ethyl acetate in toluene to obtain the compound shown in formula V or formula VI the indicated compound;
[0052]
[0053] (3) Under anhydrous and oxygen-free conditions, the compound shown in the formula V or the compound shown in the formula VI and the biboronic acid pinacol ester under the action of tetrakis (triphen...
Embodiment 1
[0080] The preparation of the near-infrared two-region fluorescent molecule containing benzobisthiadiazole in formula I:
[0081] (1) Add 11.5g of 4-bromotriphenylamine into a three-necked flask equipped with a magnet. Vacuum three times, under stirring in an ice bath, add dry 80mL N,N-dimethylformamide with a constant pressure separatory funnel, then slowly drop in 30mL phosphorus oxychloride, stir at room temperature for 1 hour, then raise the temperature to Continue the reaction at 95°C for 12 hours. After the reaction, the resulting reaction solution was cooled to room temperature, 330 mL of ice water was added, and then neutralized to neutral with 2 mol / L NaOH solution. Collect the precipitate after suction filtration, dissolve the precipitate with dichloromethane, add anhydrous sodium sulfate to remove water, and finally filter the solution, spin dry with a rotary evaporator, and separate the spin-dried product through a chromatographic column (the eluent is dichloro me...
Embodiment 2
[0086] Preparation of the near-infrared two-region fluorescent molecule containing benzobisthiadiazole in formula II:
[0087] (1) Take 1.34g of the compound of formula IV obtained in Step (1) of Example 1, 2.79g of (triphenylphosphine) ethyl acetate, add it to a 200mL three-necked flask equipped with a magnet, vacuumize three times, and then use a syringe Add 70 mL of deoxygenated toluene, stir and react at room temperature for 48 h, then cool to room temperature, spin dry with a rotary evaporator, and separate through a chromatographic column to obtain the compound represented by formula VI.
[0088] (2) 1.93g of the compound shown in formula VI obtained in step (1), 1.48g of pinacol diborate, 0.28g of tetrakis (triphenylphosphine) palladium and 1.09g of potassium acetate, add a 150mL three-port In the flask, evacuate three times, then add 60mL of deoxygenated dioxane with a syringe, the reaction temperature is 100°C, stir and reflux for 12 hours, spin dry with a rotary evap...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com