Cistanche effective ingredient extraction and enrichment method and equipment
An active ingredient, Cistanche herba technology, applied in the field of extraction and enrichment of effective ingredients of Cistanche deserticola, can solve the problems of low extraction rate, weak pigment removal ability, poor enrichment and separation effect, etc., to improve the extraction and enrichment rate, and the process is simple and feasible , the effect of low pigment content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
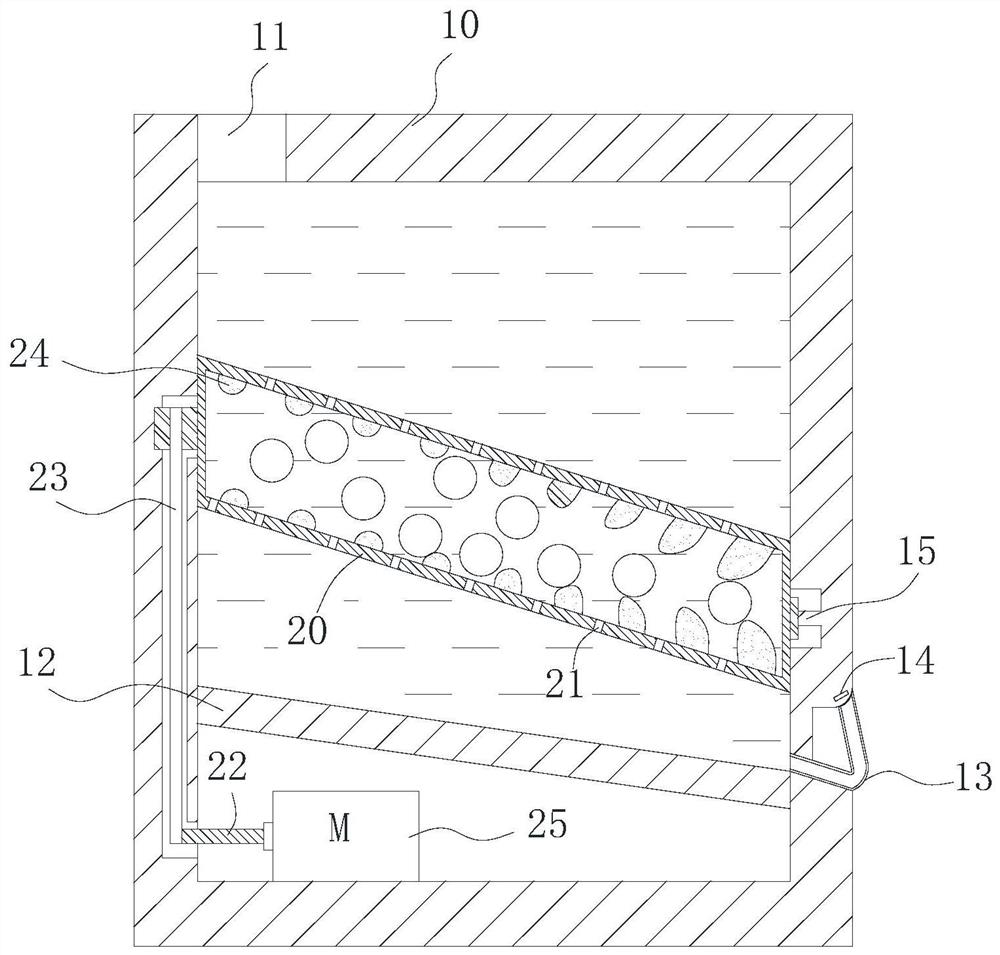
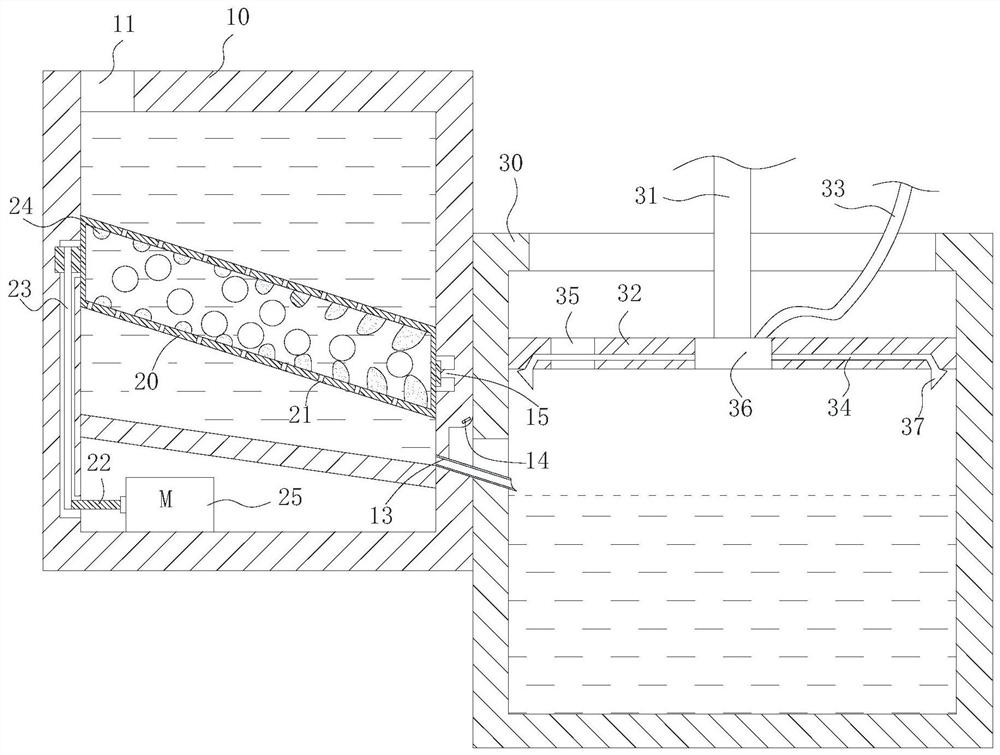
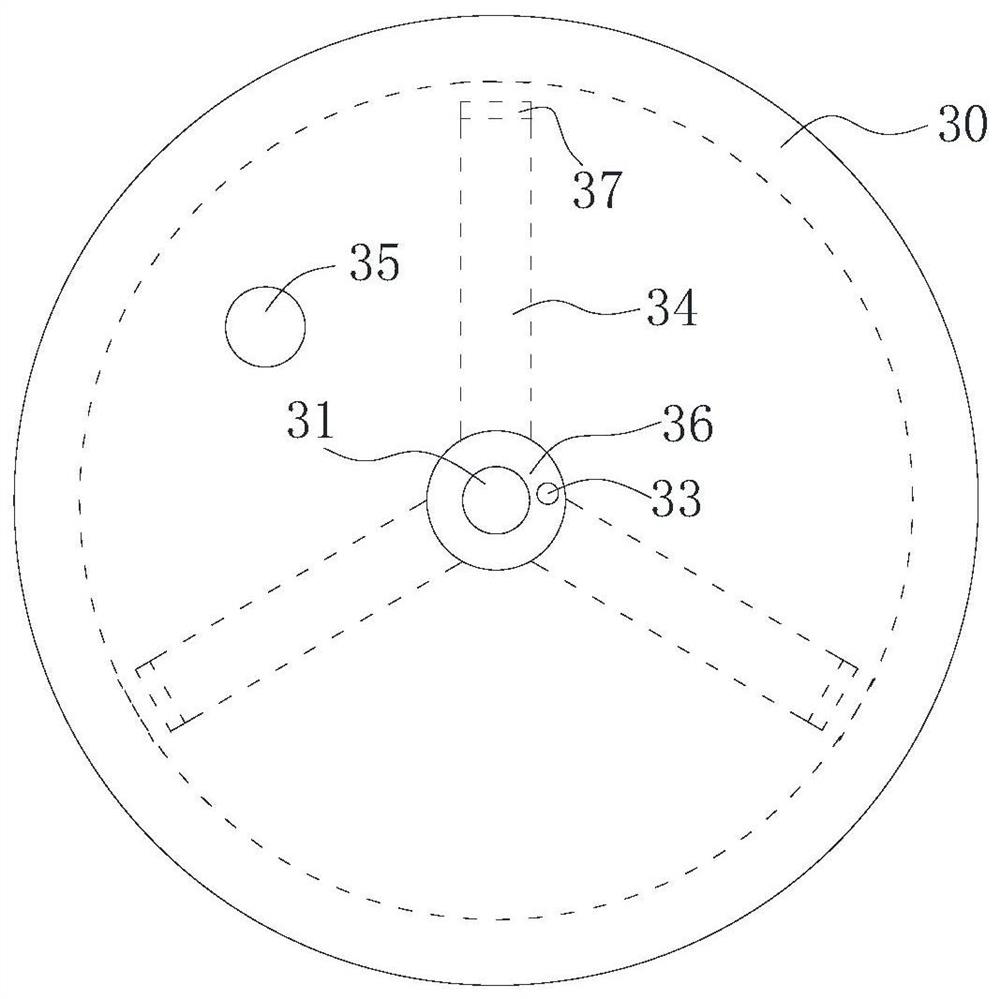
[0054] Therefore, the preferred solution is: the box cover 32 can reciprocate linearly up and down inside the concentration box 30 , and the box cover 32 is close to the inside of the concentration box 30 and fits with the inner wall of the concentration box 30 in a gap. One way for the tank cover 32 to realize the reciprocating movement is: the outside of the tank cover 32 is connected with the hydraulic rod 31 , and the reciprocating motion is performed under the action of the hydraulic rod 31 . Of course, when the amount of preparation in the laboratory is small, reciprocation can also be achieved by manpower. When needs are concentrated, first observe liquid level height through observation window, and case cover 32 descends to the position near liquid level, but does not contact with liquid level. Start heating and concentrating subsequently, along with the decline of liquid level, slowly drop case cover 32 thereupon. This maximizes ethanol recovery and concentrate colle...
Embodiment 1
[0066] Example 1: Comparison of Extraction Methods of Active Components of Herba Cistanche
[0067] 1. Dried and pulverized Cistanche medicinal materials. Weigh four parts of medicinal materials, each 3g, and place them in the extraction box 10 respectively. Two of them adopt the cold soaking method (that is, the heating device of the extraction box 10 is not started), and the ethanol aqueous solution with 12 times the weight of medicinal materials is added, and the alcoholic strength is respectively 52% vol and 70% vol. The remaining two parts adopt the heat reflux method, add ethanol aqueous solution 12 times the weight of the medicinal material, the alcohol content is 52%vol and 70%vol respectively, the temperature is 60°C, start the drum 20, stop once every 2h, collect the extract, and then add Equal amount of ethanol aqueous solution, repeated alcohol extraction under the same corresponding conditions, a total of 3 times of alcohol extraction. Through the drain pipe 13,...
Embodiment 2
[0071] Embodiment 2: single factor test of each influencing factor of Cistanche active ingredient extraction
[0072] 1. Use a single factor experiment to investigate the extraction times, solid-liquid ratio, alcohol content, time and temperature. In each group of experiments, except for variables, other constant factors are: 2g of medicinal material powder; extraction three times, 1 hour each time; temperature 75°C; mass ratio of solid to liquid 1:12; alcohol content 52% vol.
[0073] The variables of each group are: (1) temperature: 60°C, 70°C, 80°C; (2) solid-liquid ratio: 1:6, 1:8, 1:10, 1:12, 1:14; (3) time (Each time): 1h, 1.5h, 2h; (4) Alcohol: 70% vol, 52% vol.
[0074] 2, test by the method of embodiment one. The temperature test results are as follows Figure 10-12 Shown (in sequence: 60°C, 70°C, 80°C). The results of the solid-liquid ratio test are as follows: Figures 13 to 17 Shown (in sequence: 1:6, 1:8, 1:10, 1:12, 1:14). The time test results are as follo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com