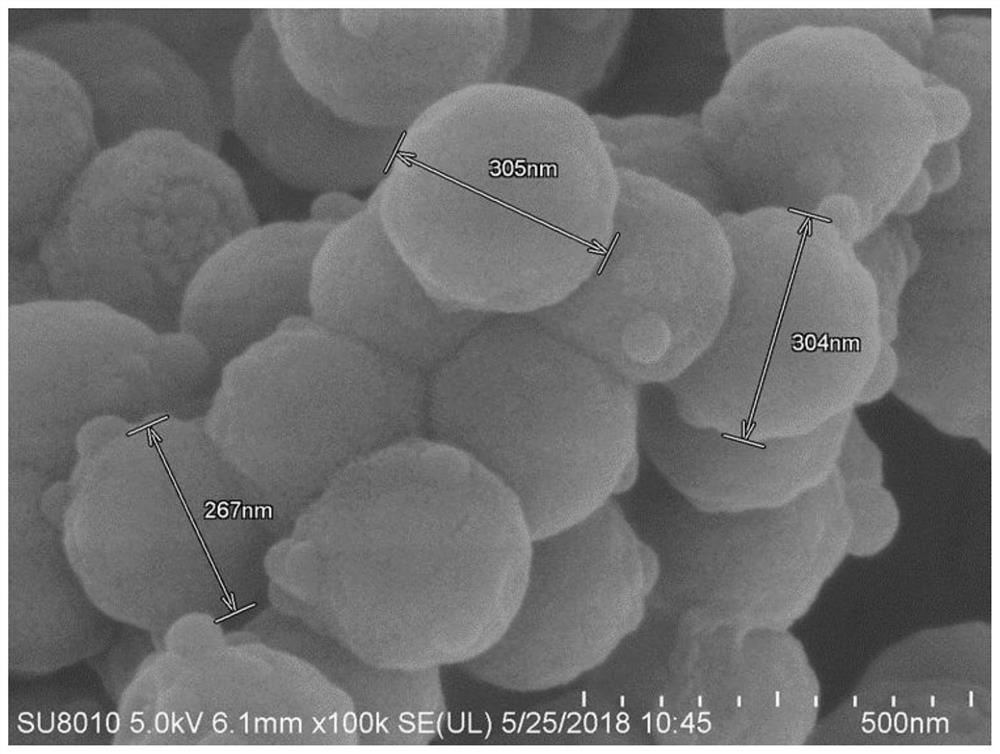
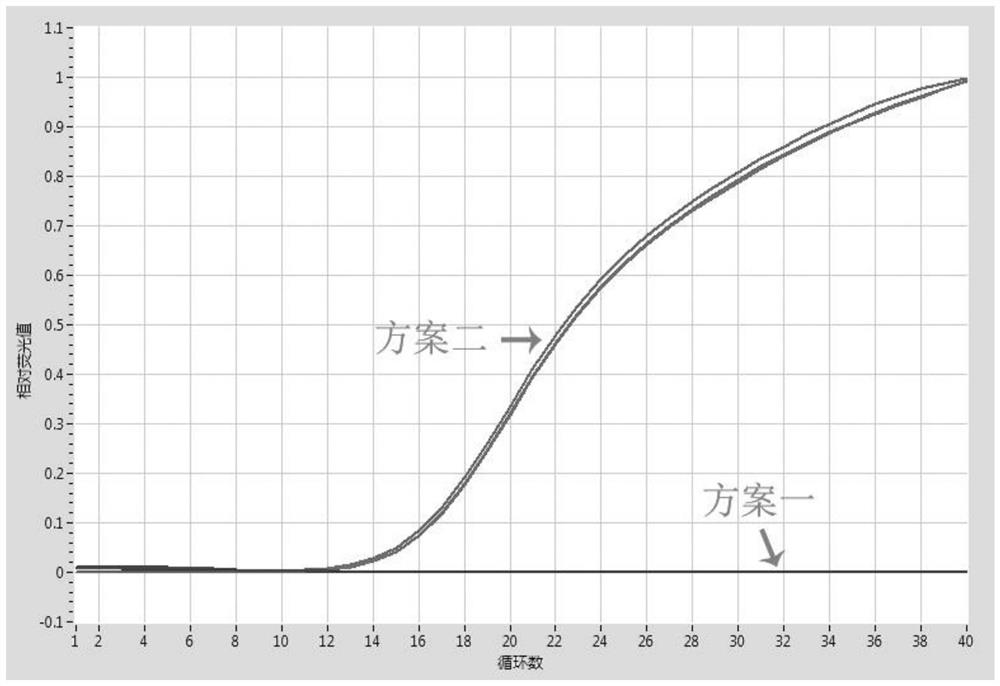
Kit for nucleic acid extraction with magnetic bead method, magnetic bead and preparation method of magnetic bead
A technology of kit and magnetic bead method, which is applied in the fields of biochemical equipment and methods, DNA preparation, microbial measurement/inspection, etc., can solve the problems of cross-contamination between samples, nucleic acid loss, damage, etc. the effect of time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0093] The sample preparation process used in the following examples is as follows:
[0094] In this example, in order to better illustrate the performance advantages of the kit in virus sample extraction, porcine transmissible gastroenteritis virus vaccine was used as the representative of RNA virus, and fowlpox virus vaccine was used as the representative of DNA virus, and prepared respectively to simulate virus infection. Serum samples, and then use the kit of this example, other comparison kits or comparison reagents to extract RNA and DNA respectively. Wherein the preparation steps of the serum sample of simulated virus infection are as follows:
[0095] Operation 1: Take 2.5mL goat serum and add it to the porcine transmissible gastroenteritis, porcine epidemic diarrhea, porcine rotavirus triple live vaccine bottle, blow and mix well, and set aside;
[0096] Operation 2: Take 2.5mL goat serum and add it to the fowlpox virus vaccine bottle, blow and mix well, and set asid...
Embodiment 1
[0107] Example 1 Preliminary optimization of cell lysate formula
[0108] In this example, two formulas of cell lysates were designed for preliminary screening of experimental schemes.
[0109] The formula of Scheme 1 is: 25mM Tris-HCl, 10mM EDTA (pH8.0), 1% SDS, 1% 3-[3-(cholamidopropyl) dimethylamino]-1-propanesulfonic acid, 0.5% N - Sodium lauroyl sarcosinate, 1M NaCl, 0.1% Antifoam204, 10% PEG6000.
[0110] The formula of scheme two is: 25mM Tris-HCl, 10mM EDTA (pH8.0), 4M guanidine isothiocyanate, 5vol% Triton X-100, 10g / L 3-[3-(cholamidopropyl) dimethyl Amino]-1-propanesulfonic acid, 5g / L sodium N-lauroyl sarcosinate, 0.28M NaCl, 0.1vol% Antifoam204, 100g / LPEG 6000.
[0111] The washing liquid formula used in this example is 20mM Tris-HCL, 0.5M NaCL, 100g / LPEG6000.
[0112] The magnetic beads of this embodiment are prepared by the following method:
[0113] a) Preparation of nanospheres: weigh 30g FeCl 3 -6H 2 0. 80g of anhydrous sodium acetate and 20g of PEG2000 w...
Embodiment 2
[0131]Embodiment 2 cell lysate formulation optimization
[0132] In order to further optimize the formulation of the cell lysate, this example is aimed at the guanidine isothiocyanate, TritonX100, PEG6000, 3-[3-(cholamidopropyl) dimethylamino]-1- The concentration of main components such as propanesulfonic acid was optimized. The optimization scheme is shown in Table 3. Formulation 9 in Table 3 will crystallize when placed at room temperature after preparation, and is not suitable for cell lysate.
[0133] Table 3 Cell lysate formulation optimization
[0134] Numbering component 1 component 2 Component 3 Component 4 Component 5 Component 6 Component 7 Component 8 Component 9 Recipe 1 2M 1vol% 0 5 5 0.28M 0.1vol% 10mM 10mM Recipe 2 2M 2vol% 100 10 5 0.28M 0.1vol% 10mM 10mM Recipe 3 2M 5vol% 200 50 5 0.28M 0.1vol% 10mM 10mM Recipe 4 3M 5vol% 0 10 5 0.28M 0.1vol% 25mM 10mM Re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com