Prostate-specific membrane antigen targeting molecular probe, preparation method and use thereof
A prostate-specific, targeting molecule technology, applied in the field of prostate-specific membrane antigen targeting molecular probes and preparation, can solve the problems of few isotopes and short half-life, and achieve the effects of high binding affinity and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
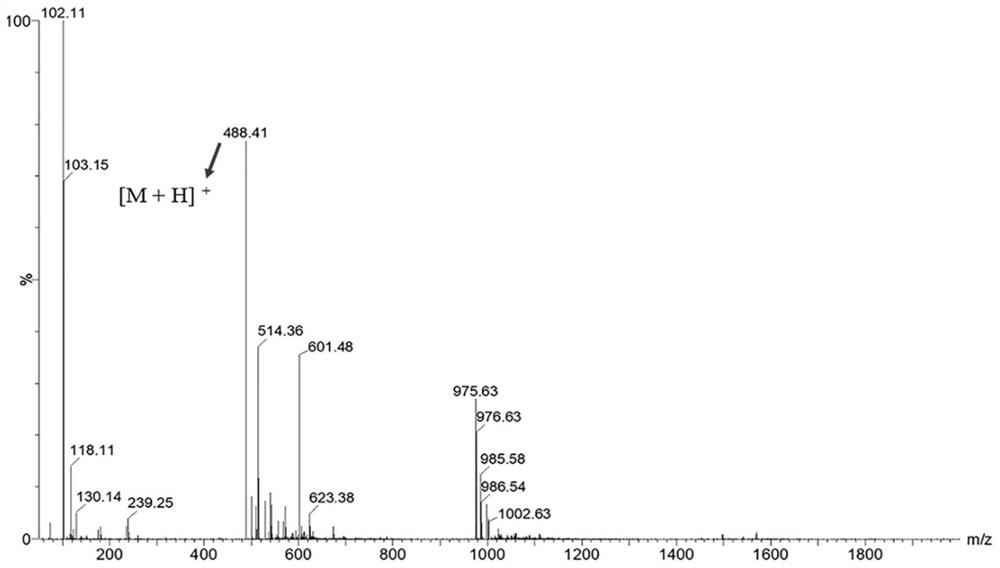
[0070] Example 1 Preparation of Prostate Specific Membrane Antigen Targeting Molecular Probe Precursor
[0071] This embodiment provides a prostate specific membrane antigen targeting molecular probe precursor, which has the structure shown in the following formula:
[0072]
[0073] The preparation method is as follows:
[0074] (1) The synthetic route of compound 4 is as follows:
[0075]
[0076] In a round bottom flask (100 mL), compound 1-4 (di-tert-butyl L-glutamate hydrochloride) (500 mg, 1.7 mmol) was dissolved in dichloromethane (20 mL), triethylamine (0.6 mL ) and -dimethylaminopyridine (8mg) in suspension. N'N-carbonyldiimidazole (300 mg, 1.86 mmol) was added to the reaction mixture and stirred at room temperature overnight. The reaction completed mixture was diluted with dichloromethane (20 mL), and extracted from saturated sodium chloride solution. After the extraction was complete, the organic phase was dried over anhydrous sodium sulfate and concentrat...
Embodiment 2
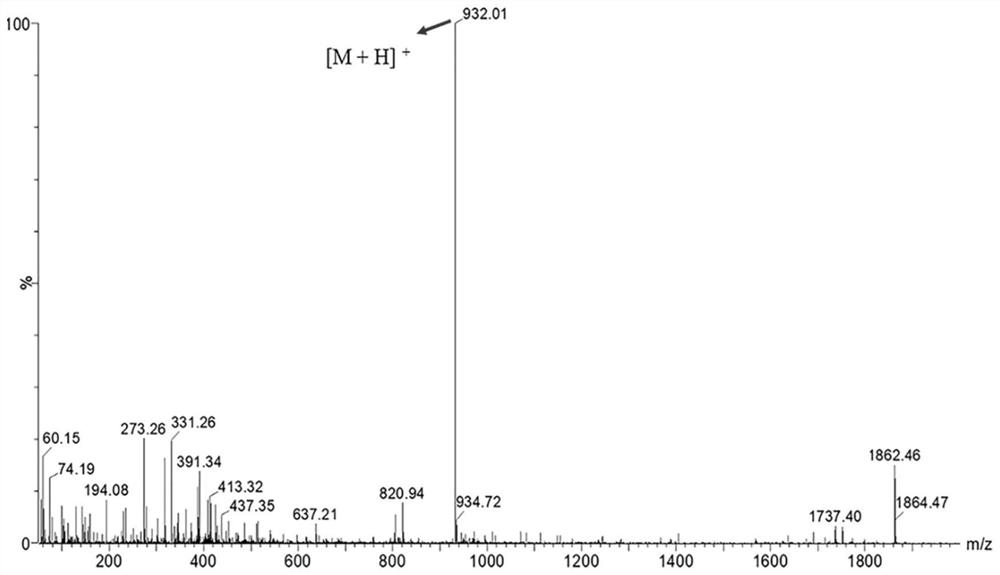
[0096] Example 2 Prostate-specific membrane antigen targeting molecular probe
[0097] This embodiment provides the prostate-specific membrane antigen targeting molecular probe [ 18 F]GLNTGT, has the structure shown in the following formula:
[0098]
[0099] The synthetic route is as follows:
[0100]
[0101] Obtain 7.4GBq of [ 18 F] Fluoride, bombarded with 18 MeV protons at high voltage [ 18 O] Water targets and rinsed with pyridazine-HCl Buffer (pH=2.5, 1 M, 300 μL) was placed directly into a centrifuge tube containing the non-radioactive compound GLNTGT (25 mM, 30 μL). The reaction mixture was incubated at 80°C for 35 minutes. Radioactive HPLC detected [ 18 F] Successful flagging of GLNTGT (results such as Figure 8 middle line B). will mark the [ 18F] GLNTGT reaction mixture was transferred to a 40 mL centrifuge tube with 20 mL deionized water. The RP-C18 chromatographic column was washed twice with the mixture in the centrifuge tube (10mL / time). The res...
experiment example 1
[0104] Experimental Example 1 Serum Stability
[0105] With the tracer prepared in embodiment 2 [ 18 F] GLNTGT (20 μL, 0.074 MBq) was added to separate centrifuge tubes containing fetal bovine serum (90 μL) and sterile phosphate-buffered saline (PBS, pH=7.4) (90 μL), respectively. The centrifuge tubes were then incubated at 37 °C for 1 h, 2 h and 4 h. 10 μL of the incubation samples were collected at each time point, and then 10 μL of acetonitrile were added to each of them to precipitate serum proteins. The supernatant for radio-HPLC analysis was obtained by centrifuging the incubated samples (12000 rpm, 1 min). The radiation-HPLC analysis method of the supernatant is shown in Table 3 in Example 2.
[0106] The result is as Figure 8 As shown (lines D, E, F and G in the figure), during the 4-hour incubation, [ 18 F] GLNTGT is always stable in PBS and fetal bovine serum.
[0107] Experimental Example 2 Lipophilicity
[0108] Determination of the tracer by the shake flas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com