Preparation method of brivaracetam intermediate
A compound and reaction technology, applied in the field of preparation of brivaracetam intermediates, can solve the problems of complicated synthesis process operation, high production cost, difficult realization and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
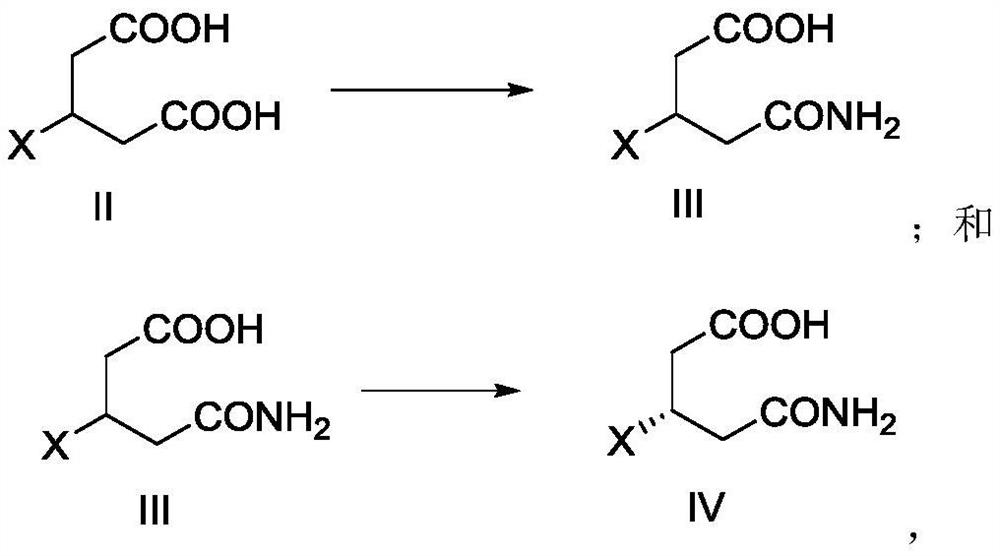
[0084] Example 1 Preparation of Compound III-A
[0085]
[0086] Take compound II-a (3-propylglycidic acid) (10.0 g, 1.0eq) and urea (3.4 g, 1.0eq), mixed, stirred in an oil bath of 80 ° C for about 12 h, then cool down to about 40 ° C, add NaOH (2.3 g, 1.0 eq) and water (20 mL) NaOH aqueous solution, stirred at 80 ° C for 3 h; cooling to room temperature, adding hydrochloric acid solution containing concentrated hydrochloric acid (9.9 g) and water (38 mL), and continued The slow temperature elimination was stirred at 5 ° C and stirred for 1 h; filtered, the filter cake was washed with 1N dilute hydrochloric acid (5 mL), and the vacuum dried at 50 ° C to obtain a compound III-A: white solid, 9.1 g, yield of 92%; The resulting compound III-A was detected by hydrogen spectrum, carbon spectrum and mass spectrometry, as follows:
[0087] 1 H NMR (400 MHz, DMSO) δ12.04 (S, 1H), 7.33 (S, 1H), 6.80 (S, 1H), 2.24 (S, 4H), 0.82 (T, J = 5.9 Hz, 3H);
[0088] 13 C NMR (151 MHz, DMSO) Δ174....
Embodiment 2
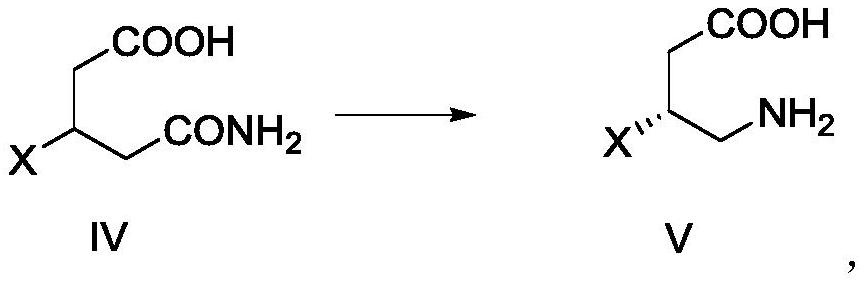
[0090] Example 2 Preparation of Compound IV-A
[0091]
[0092] Compound III-a (7.8 g, 1.0 eq), S - (-) - α-phenylamine (4.0 g, 0.72 eq), ethanol (2.4 g, 1.12eq) and DCM (150 ml), mix, 50 ° C The mixture was stirred for about 2.5 h, cooled to 25 ° C, stirred for 2 h; then continued to cool down to 0 ° C for 12 h, there was solid precipitation, filtration, filter cake was washed with 50 ml of DCM (0 ° C cooling treatment), 50 ° C drying 12h, resulting shallow Red solid; add 50 ml of water, stirred under 30 ° C for solid dissolution; cool down to 20 ° C, add concentrated hydrochloric acid (3.6 g) and water (10 mL) hydrochloric acid solution, there is solid precipitation, stirring is stirred after 30min, cool down After 5 ° C, it continued to be pulp for 1 h; filtration, the filter cake was washed once with concentrated hydrochloric acid (2.0 g) and aqueous solution (10 mL), and dried at 50 ° C for 12 h to give Compound IV-A: white solid, 3.6g, The yield was 46%; the obtained compo...
Embodiment 3
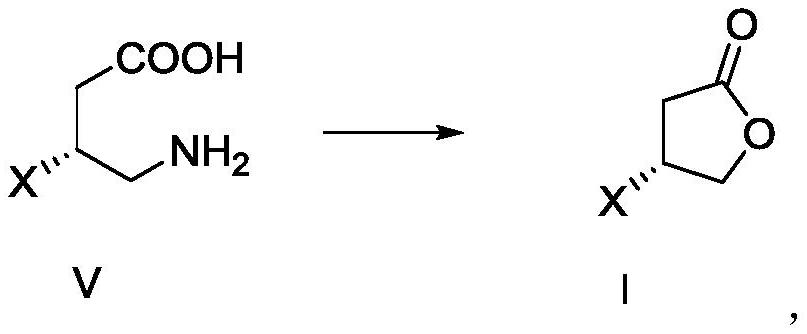
[0096] Example 3 Preparation of Compound V-A
[0097]
[0098] The compound IV-A (5.0 g, 1.0 eq) and NaOH (1.2 g, 1.0 eq) and water (6 ml) NaOH aqueous solution were mixed, stirred at 25 ° C to dissolve, cooled to about 0 ° C, and NaOH (2.1) g, 1.8 eq) and water (4 mL) NaOH aqueous NaOH aqueous solution and sodium hypochlorite solution (22.5 g, 10% effective chlorine) mixed solution continued at 0 ° C for 1.5 h; 30 ° C for 12 h; cool down to 20 ° C, drop 12N thick Hydrochloric acid (13.0 g), solid production, yellow green, 0.5 g of NaOH (2.5 g) and water (5 mL) NaOH aqueous solution, adjusting pH to 7 ~ 8, solid loss; concentrated to have solid precipitation, Covered to 0 ° C to be pulp 3H; filtration, filter cake was dried in vacuo to 50 ° C for 12 h, resulting in a compound VA: white solid, 4.0 g, yield of 93%; to take an appropriate amount of compound VA to detect hydrogen spectrum, carbon spectrum and mass spectrometry, as follows:
[0099] 1 H NMR (400MHz, D 2 O) δ3.32 (DDD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com