Mavacamten crystal form I and preparation method thereof
A crystal form, cu-k technology, applied in the field of crystal form I of Mavacamten, a new drug for the treatment of hypertrophic cardiomyopathy, and its preparation, can solve the problem that there is no report of Mavacamten crystal form, which affects drug stability, bioavailability, curative effect, and dissolution rate , bioavailability and other issues, to achieve the effect of simple preparation process, easy operation and easy storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add Mavacamten (1.0g, purity 98.0%) and 5mL of anhydrous methanol into a three-necked flask, stir evenly, heat up to 55-60°C, stir for 4-6 hours, slowly cool to 0-5°C for crystallization, and keep stirring for 16 After ~24 hours, centrifuge and dry to obtain 0.81 g of Form I (purity 99.9%, yield 82.6%).
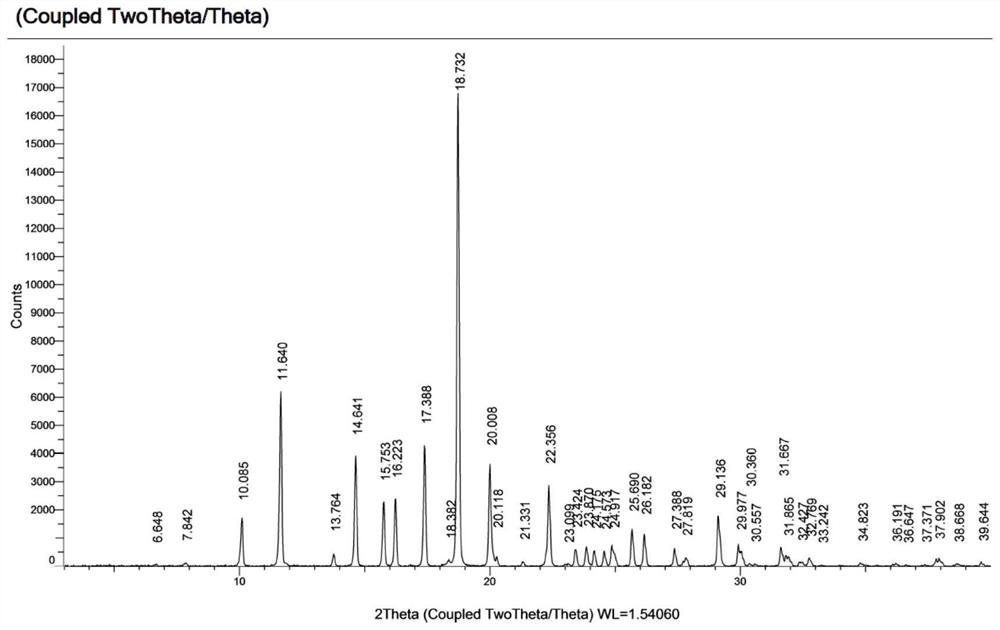
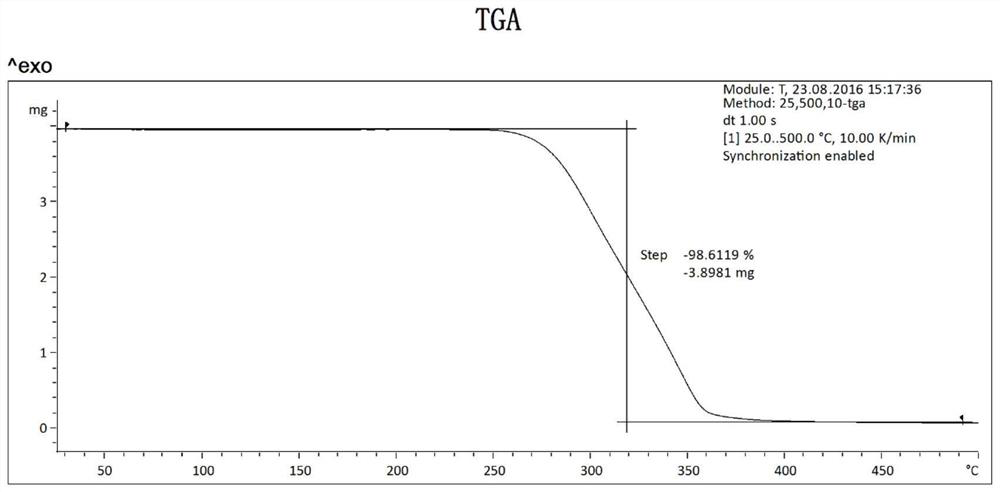
[0036] The X-ray powder diffraction data of the crystal form I obtained in this embodiment are shown in Table 1, and its XRPD pattern is as follows figure 1 As shown, its DSC diagram is shown in figure 2 As shown, its TGA diagram is shown in image 3 shown.
[0037] Table 1
[0038]
[0039]
[0040] Anhydrous methanol can be used ethanol, isopropanol, n-butanol, ethyl acetate, isopropyl acetate, dichloromethane, tetrahydrofuran, 2-methyltetrahydrofuran, N,N-dimethylformamide, N , N-dimethylacetamide, dimethyl sulfoxide, N-methylpyrrolidone, acetone or acetonitrile instead; the amount of methanol is feasible within the range of 0.5 ~ 40ml.
Embodiment 2
[0042] Add Mavacamten (1.0g, purity 98.0%) and 10mL of absolute ethanol into a three-necked flask, stir well, heat up to 55-60°C, stir for 4-6 hours, slowly cool to 0-5°C for crystallization, and keep stirring for 16 After ~24 hours, centrifuge and dry to obtain 0.86 g of Form I (purity 99.8%, yield 87.6%).
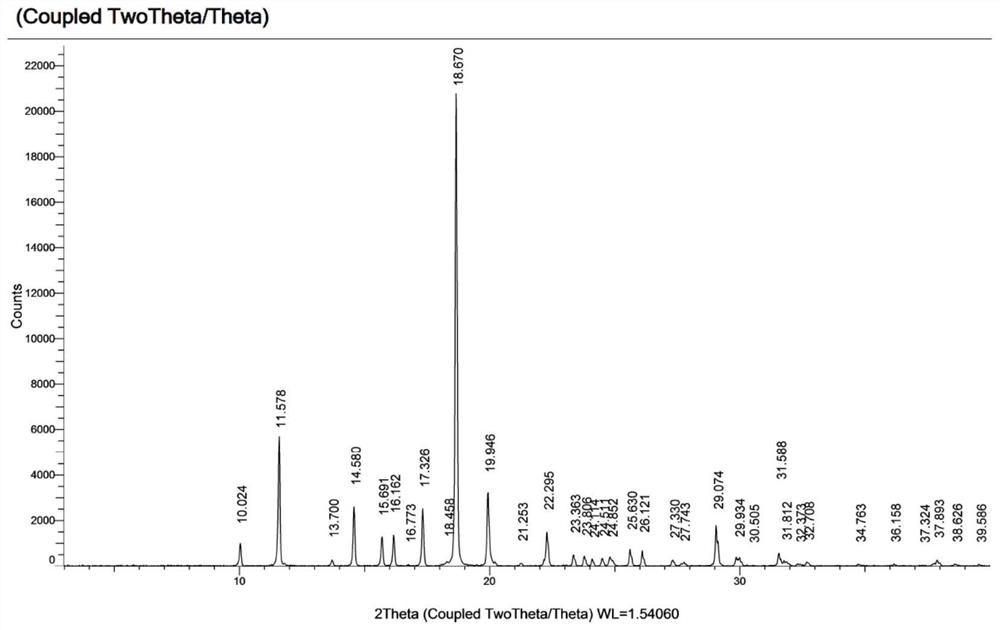
[0043] Table 2 shows the X-ray powder diffraction data of Form I obtained in this example.
[0044] Table 2
[0045]
[0046]
[0047] Dehydrated alcohol can be available methanol, isopropanol, n-butanol, ethyl acetate, isopropyl acetate, dichloromethane, tetrahydrofuran, 2-methyltetrahydrofuran, N,N-dimethylformamide, N , N-dimethylacetamide, dimethyl sulfoxide, N-methylpyrrolidone, acetone or acetonitrile instead; the amount of ethanol is feasible within the range of 0.5-40ml.
Embodiment 3
[0049] Add Mavacamten (1.0 g, purity 98.0%) and 10 mL of isopropanol into a three-necked flask, stir evenly, heat up to 55-60°C, stir for 4-6 hours, slowly cool to 0-5°C for crystallization, and keep stirring for 16 After ~24 hours, centrifuge and dry to obtain 0.91 g of Form I (purity 99.8%, yield 92.7%).
[0050] Table 3 shows the X-ray powder diffraction data of Form I obtained in this example.
[0051] table 3
[0052]
[0053]
[0054] Virahol can be used ethanol, methanol, n-butanol, ethyl acetate, isopropyl acetate, dichloromethane, tetrahydrofuran, 2-methyltetrahydrofuran, N,N-dimethylformamide, N,N - Dimethylacetamide, dimethyl sulfoxide, N-methylpyrrolidone, acetone or acetonitrile instead; the amount of isopropanol is feasible within the range of 0.5-40ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com