Iodine oil emulsion or drug-loaded iodine oil emulsion as well as preparation method and application thereof
A technology of iodized oil emulsion and iodized oil, which is applied in the directions of medical formula, X-ray contrast agent preparation, drug combination, etc., can solve problems such as ischemic necrosis or ectopic embolism, and achieve the effect of expanding the indications of iodized oil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1 adopts ether to inject-ultrasonic emulsification method to prepare lipiodol emulsion
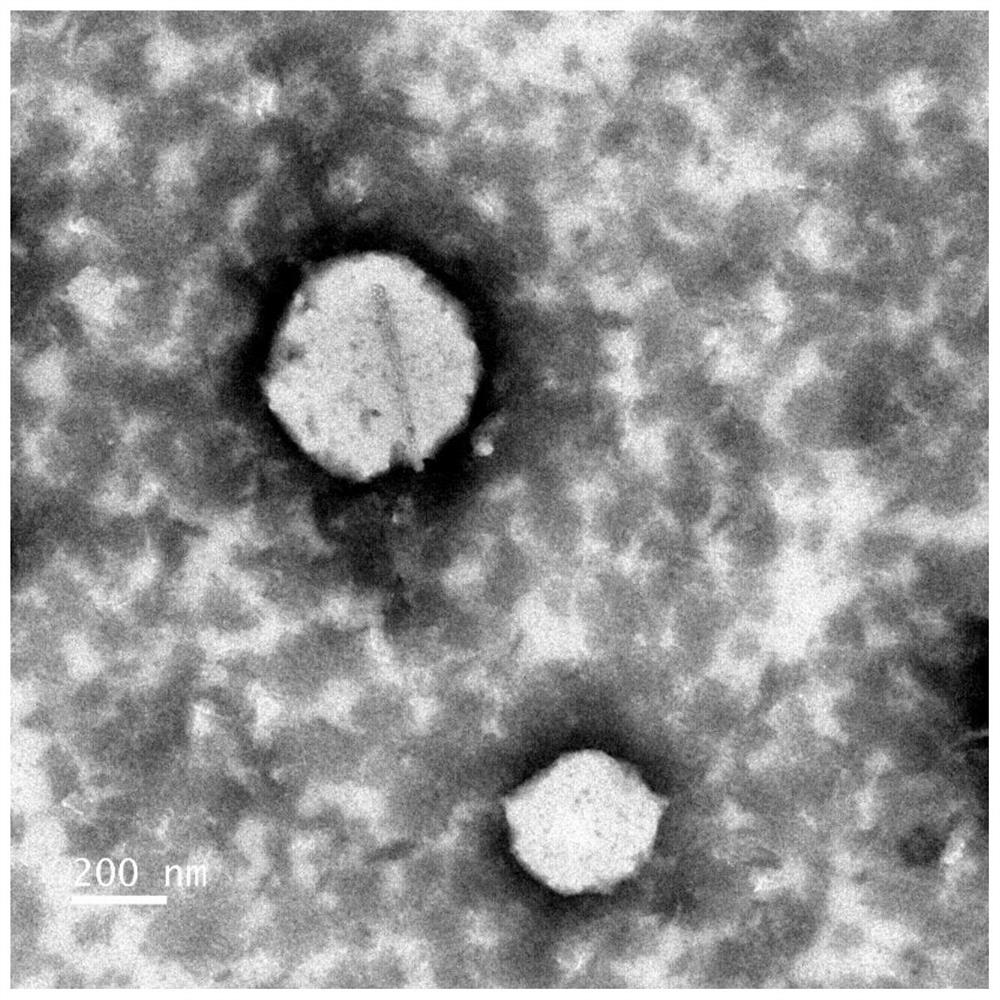
[0060] Weigh 1,320 mg of soybean lecithin and 220 mg of cholesterol into a beaker, add 200 μL of iodized oil (National Drug Approval H37022398) and 4 ml of absolute ethanol, and place at 50°C to fully dissolve. Pipette the dissolved lipid solution evenly and pour it into 6ml of phosphate buffer solution pH6.0, then transfer it to an eggplant-shaped bottle, and place it in a water bath at 50-60°C to remove ether by vacuum rotary evaporation. The vacuum degree is - 0.08MPa, treatment time 20min, transferred to a centrifuge tube or beaker containing 200mg Tween-80, and then ultrasonicated at 240W for 24min until a uniform emulsion was formed. See figure 1 . Obtaining the emulsion that iodine content is 9.6mg / ml, electron micrograph and particle size distribution see respectively figure 2 with image 3 . The emulsion is stored in a 4°C refrigerator for 12 months, and it...
Embodiment 2
[0062] Example 2 Preparation of Lipiodol-Mesylate Apatinib Nano-liposomes Using Ethanol Injection-Ultrasonic Method
[0063] Weigh 660 mg of soybean lecithin and 55 mg of cholesterol into a beaker, add 500 μL of super liquefied lipiodol, and 5 ml of absolute ethanol at 45°C to fully dissolve. Inject the dissolved lipid solution into 50ml of phosphate buffer (concentrated hydrochloric acid adjusted to pH 2) containing 500mg of apatinib mesylate, then transfer it to an eggplant-shaped bottle, place it in a water bath at 45°C under vacuum Remove ethanol by rotary evaporation, vacuum degree -0.08MPa, treatment time 20min, transfer to a centrifuge tube or beaker containing 5mg Tween-80, and then sonicate at 141W for 24min until a uniform emulsion is formed. Check the pH and adjust the pH to 7 with 10% NaOH. A homogeneous emulsion with iodine content of 4.8 mg / ml and apatinib content of 10 mg / ml was obtained. See Image 6 . Obtaining the emulsion that iodine content is 9.6mg / ml,...
Embodiment 3
[0065] Example 3 Preparation of Lipiodol-Adriamycin Liposomes Using Ethanol-DMSO Injection-Ultrasonic Method
[0066] Weigh 660mg of soybean lecithin and 220mg of cholesterol into a beaker, add 20mg of doxorubicin hydrochloride, add 1ml of lipiodol, 200mg of Tween-80, and 5ml of absolute ethanol, place at 50°C to fully dissolve. Inject the dissolved lipid solution into 9ml of phosphate buffer solution pH6.6 (0.05mol / L), then transfer it to an eggplant-shaped bottle, and place it in a water bath at 79°C for vacuum rotary evaporation to remove ethanol and dimethyl Sulfoxide, the vacuum degree is -0.1MPa, the treatment time is 20min, transferred to a 50ml centrifuge tube or beaker, and then ultrasonicated at 141W for 20min until a uniform emulsion is formed. See Figure 10 , the transmission electron microscope observation picture is shown in Figure 11 .
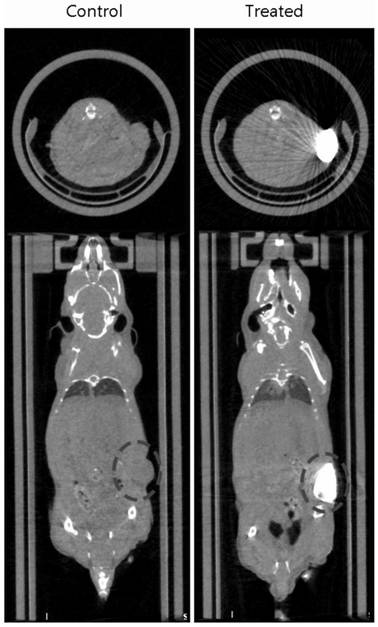
[0067] The animal experiment is as claimed in claim 1, using h22 cells to subcutaneously plant tumors in ICR mice, record...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com